A 12.5-L scuba diving tank contains a heliumoxygen (heliox) mixture made up of 24.2 g of He
Question:
A 12.5-L scuba diving tank contains a helium–oxygen (heliox) mixture made up of 24.2 g of He and 4.32 g of O2 at 298 K. Calculate the mole fraction and partial pressure of each component in the mixture and the total pressure of the mixture.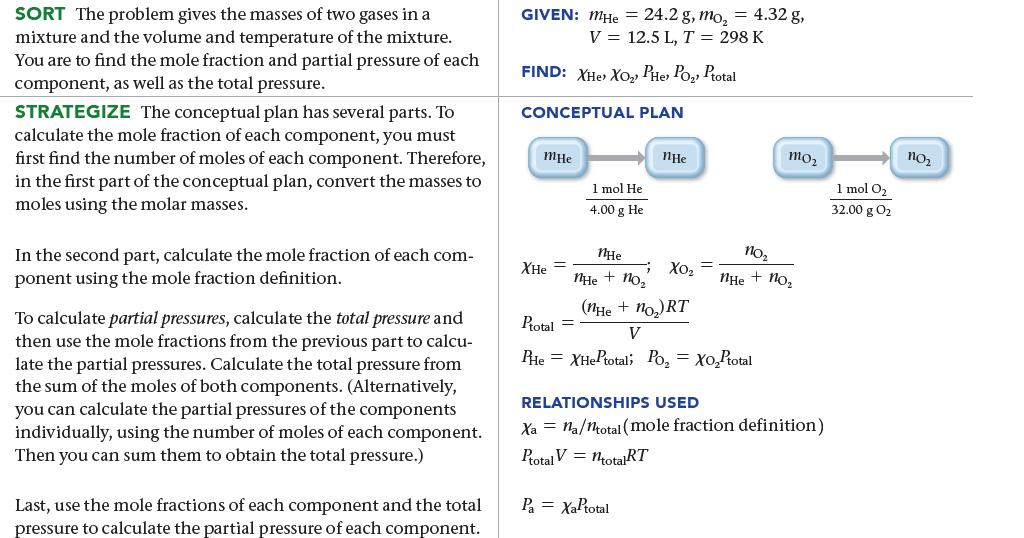
Transcribed Image Text:
SORT The problem gives the masses of two gases in a mixture and the volume and temperature of the mixture. You are to find the mole fraction and partial pressure of each component, as well as the total pressure. STRATEGIZE The conceptual plan has several parts. To calculate the mole fraction of each component, you must first find the number of moles of each component. Therefore, in the first part of the conceptual plan, convert the masses to moles using the molar masses. In the second part, calculate the mole fraction of each com- ponent using the mole fraction definition. To calculate partial pressures, calculate the total pressure and then use the mole fractions from the previous part to calcu- late the partial pressures. Calculate the total pressure from the sum of the moles of both components. (Alternatively, you can calculate the partial pressures of the components individually, using the number of moles of each component. Then you can sum them to obtain the total pressure.) Last, use the mole fractions of each component and the total pressure to calculate the partial pressure of each component. GIVEN: MHe = 24.2 g, mo₂ = 4.32 g, V = 12.5 L, T = 298 K FIND: XHe> XO₂ PHe› Po₂) Ptotal CONCEPTUAL PLAN mHe 1 mol He 4.00 g He XHe nHe 9 XO₂ nHe nHe + no₂ (nHe + no₂) RT Ptotal = V PHe XHEPtotal; Po₂ = XO₂Ptotal Pa = XaPtotal mo₂ no₂ nHe + no₂ RELATIONSHIPS USED Xa na/ntotal (mole fraction definition) Ptotal V = totalRT 1 mol O₂ 32.00 g 0₂ no₂
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
242 g He x 432 g 0 x XHe XO Ptotal 1 mol He 400 g He 1 mol O 3200 g ...View the full answer

Answered By

Shristi Singh
A freshman year metallurgy and material science student in India.
4.80+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A solution contains 3.95 g of carbon disulfide (CS 2 ) and 2.43 g of acetone (CH 3 COCH 3 ). At 35 C the vapor pressures of pure carbon disulfide and pure acetone are 515 torr and 332 torr,...
-
1 723 Conditions for promotions 5 Years of service (Years) 6 Psychometric test (%) Required: a) LIST OF EMPLOYEES FOR PROMOTION 9 Names of employees Years of service 10 Munawarah Ali 11 Amiruddin...
-
Calculate the mole fraction of each component and the gas constant of the mixture for each of the following mixtures: a) 4 kg N2, 1 kg O2, 3 kg CO2 b) 4 kg N2, 1 kg CH2, 3 kg NH3 c) 5 kg air, 3 kg...
-
A decline in the aggregate demand has led to a decline in output in the economy equal to $10 billion. Government wants to counter this recession by reducing income taxes. Assuming that the marginal...
-
Describe some important uses of electronic commerce and explain why it is important to accountants.
-
What is the first iteration in the elaboration phase?
-
State the defining characteristics of six types of organizational structures used in the hospitality industry as they relate to: Taxes Liability Financing Transfer of ownership AppendixLO1
-
The figure shows a shaft mounted in bearings at A and D and having pulleys at B and C. The forces shown acting on the pulley surfaces represent the belt tensions. The shaft is to be made of ASTM...
-
Clown Co. ordered parts costing 200,000 Foreign Currency Units (FCU) from a foreign supplier on October 1 when the spot rate was $0.27 per FCU. A one-month forward contract was signed on that date to...
-
The crank OA rotates in the vertical plane with a constant clockwise angular velocity w 0 of 4.5 rad/s. For the position where OA is horizontal, calculate the force under the light roller B of the...
-
Aluminum reacts with chlorine gas to form aluminum chloride. What minimum volume of chlorine gas (at 298 K and 225 mmHg) is required to completely react with 7.85 g of aluminum? a) 36.0 L b) 24.0 L...
-
The graph shows PV/RT for carbon dioxide at three different temperatures. Rank the curves in order of increasing temperature. (a) C (b) A (c) B (d) C PV/RT 2. 1.6- 1.2- 0.8- 0.4- 0- 0 200 400 600...
-
Tisha Allen opened Tishas Cosmetic Market on December 1. An 8% sales tax is calculated and added to all cosmetic sales. Tishas offers no sales discounts. The following transactions occurred in...
-
From your reading this unit on motivation and change from the TIP series, what is the connection and interplay between these concepts/statements below in your opinion in working with clients facing...
-
Please help with the following The partnership of Bauer, Ohtani, and Souza has elected to cease all operations and liquidate its business property. A balance sheet drawn up at this time shows the...
-
Pacifico Company, a U.S.-based importer of beer and wine, purchased 1,200 cases of Oktoberfest-style beer from a German supplier for 276,000 euros. Relevant U.S. dollar exchange rates for the euro...
-
Define meaning of partnership deed.
-
List down the information contains in the partnership deed.
-
Name the micro structural products of eutectoid iron-carbon alloy (0.76 wt% C) specimens that are first completely transformed to austenite, then cooled to room temperature at the following rates:...
-
If someone's Z-score for a variable was 0.67. Their score is a significant extreme score. Their score is not significant. O Their score is slightly above average. O Their score is an outlier.
-
Determine the moments at D and C, then draw the moment diagram for each member of the frame. Assume the supports at A and B are pins and D and C are fixed joints. EI is constant. 5 k/ft 12 ft- 9 ft A
-
Determine the reactions at A and D. Assume the supports at A and D are fixed and B and C are fixed connected. EI is constant. 8k/ft 15 ft -24 ft-
-
Determine the moments at the ends of each member of the frame. Assume the joint at B is fixed, C is pinned, and A is fixed. The moment of inertia of each member is listed in the figure. E = 29(10 3 )...
-
If a company has an opportunity to invest $85000 now for 14 years at 18% per year simple interest or 10% per year compound interest, which investment should be made?
-
A taxpayer can claim a personal exemption for any person he or she provides over 50 percent of support during the tax year. True False
-
Holding other variables constant, a recession in the US economy should: Select one: A) cause a depreciation of the dollar because the nominal interest rate in the United States would increase b)...

Study smarter with the SolutionInn App


