A Lewis structure for the acetate ion is shown here: Which structure is the best resonance structure
Question:
A Lewis structure for the acetate ion is shown here: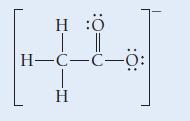
Which structure is the best resonance structure for the acetate ion?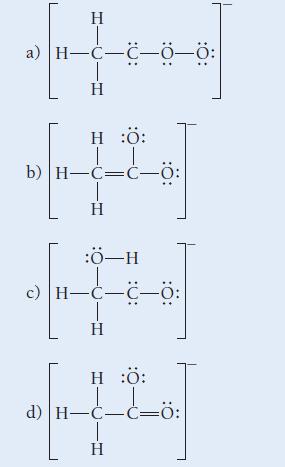
Transcribed Image Text:
HÖ | || H-C-C-Ö: H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
H L...View the full answer

Answered By

Amit Kumar
I am a student at IIT Kanpur , which is one of the prestigious colleges in INDIA.
Cleared JEE Advance in 2017.I am a flexible teacher because I understand that all students learn in different ways and at different paces. When teaching, I make sure that every student has a grasp of the subject before moving on.
I will help student to get the basic understanding clear. I believe friendly behavior with student can help both the student and the teacher.
I love science and my students do the same.
4.90+
44+ Reviews
166+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Draw the Lewis structure (including resonance structures) for the acetate ion (CH 3 COO ). For each resonance structure, assign formal charges to all atoms that have formal charge. Formal charge =...
-
The "plastic" explosive C-4, often used in action movies, contains the molecule cyclotrimethylenetrinitramine, which is often called RDX (for Royal Demolition eXplosive):...
-
A second Lewis structure can be drawn for one of the nucleophiles in Problem 36. (a) Identify it and draw its alternate structure (which is simply a second resonance form), (b) Is there a second...
-
Technology World tsad the following revenue and expenses during the month ended July 31, Fees for computer repairs Advertising expense Salaries expense Telephone expenses fees for printer repairs...
-
Aspen Products, Inc., began production of a new product on April 1. The company uses a standard cost system and has established the following standards for one unit of the new product: During April,...
-
Z Ltd purchased a retail store and commenced business on April 1. From the following information, you are required to prepare in as much details as possible, a trading and profit and loss account for...
-
Understand the differences between mediation and negotiation.
-
Theo Harris earns $55,000 a year and has $9,000 to invest in a portfolio. His investment alternatives and their expected returns are shown in the table at the top of the next column. Theos investment...
-
ThreePoint Sports Inc, manufactures basketballs for the Women's National Basketball Association (WNBA). For the first 6 months of 2020, the company reported the following operating results while...
-
1. Display the age and name for the pilot who was the oldest when HIRED. 2. Using a SET OPERATOR, display the pilots and the number of miles flown as "Miles Flown", include pilots who have not yet...
-
How does lattice energy relate to ionic radii? To ion charge?
-
What is the BornHaber cycle? List each step in the cycle and show how the cycle is used to calculate lattice energy.
-
Standard costs facilitate management planning. What are the other advantages of standard costs?
-
1. A large group of students were asked what their favorite soft drink is. Below is the probability distribution for a student chosen at random liking a particular soft drink. Drink: Choka Kola CR...
-
Task: Identify a local (within 50km of North Bay) business and answer the following questions: Name of Business: 1. Is the business independent or is it a chain? What is one advantage of this...
-
What questions would you like to ask of Cassie to better understand any factors that may be affecting Sasha at this time? Growing sunflowers It's now week 6 into the growing sunflowers project. Your...
-
n rope is fixed to a wall and attached to the block such that the rope is parallel to the surface of the wedge. The 12 points) Consider the situation in the figure where a square block (mi) sits...
-
A Zn/Zn2+ concentration cell is constructed in which both electrodes are pure zinc. The Zn2+ concentration for one cell half is 1.0 M, for the other, 10-2 M. Is a voltage generated between the two...
-
A number of years ago the United Food and Commercial Workers Union organized 800 workers of the 1035 employees at one of the Wilson Brothers food operations in Toronto, Ontario. The employees include...
-
The column is built up by gluing the two boards together. If the wood has an allowable normal stress of Ï allow = 6 MPa, determine the maximum allowable eccentric force P that can be applied to...
-
The column is built up by gluing the two boards together. Determine the maximum normal stress on the cross section when the eccentric force of P = 50 kN is applied. 150 mm 250 mm 75 mm 150 mm 50 mm...
-
The steel bracket is used to connect the ends of two cables. If the applied force P = 1.50 kip, determine the maximum normal stress in the bracket. Assume the bracket is a rod having a diameter of...
-
What is the price of a five-year bond with an 8% coupon and a 10% yield to maturity assuming annual coupon payments
-
CROSS RATES Suppose the exchange rate between the U.S. dollar and the Swedish krona was 6 krona = $1, and the exchange rate between the dollar and the British pound was 1 = $1.85. What would be the...
-
0 Suppose that two different studies (A and B) have the same sample sizes in e four groups, with similar standard deviations in the four groups. Furthermore sample sizes and sample SDs are also the...

Study smarter with the SolutionInn App


