A solution is 0.0250 M in Pb 2+ . What minimum concentration of Cl is required
Question:
A solution is 0.0250 M in Pb2+. What minimum concentration of Cl– is required to begin to precipitate PbCl2?
For PbCl2, Ksp = 1.17 * 10-5.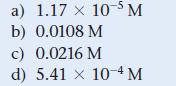
Transcribed Image Text:
a) 1.17 x 10-5 M b) 0.0108 M c) 0.0216 M d) 5.41 x 10-4 M
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
c...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
What is the Cl concentration just as Ag2CrO4 begins to precipitate when 1.0 MAgNO3 is slowly added to a solution containing 0.015 M Cl and 0.015 M CrO42?
-
Discuss how laws and regulations guide total compensation. Research a law or regulation and discuss how it influences total compensation in your organization or an organization you are familiar with....
-
A solution of Na2SO4 is added drop wise to a solution that is 0.010 M in Ba2+ and 0.010 M in Sr2+. (a) What concentration of SO42- is necessary to begin precipitation? (Neglect volume changes. BaSO4...
-
Find the point on the plane z = x + y + 1 closest to the point P = (1, 0, 0). Minimize the square of the distance.
-
Gator Corporation manufactures several types of accessories. For the year, the gloves and mittens line had sales of $500,000, variable expenses of $370,000, and fixed expenses of $150,000. Therefore,...
-
It The wavelengths of light emitted by a firefly span the visible spectrum but have maximum intensity near \(550 \mathrm{~nm}\). A typical flash lasts for \(100 \mathrm{~ms}\) and has a power of...
-
The importance of cross-validation
-
On January 1, 2016, Sayers Company issued $280,000 of five-year, 6 percent bonds at 102. Interest is payable semiannually on June 30 and December 31. The premium is amortized using the straight-line...
-
(a) What does "Parent Company Perspective" and "Project Perspective" mean? State what evaluative technique they relate to and define both. (b) Briefly describe how the computation of these 2...
-
Which compound is more soluble in an acidic solution than in a neutral solution? a) PbBr 2 b) CuCl c) AgI d) BaF 2
-
You add potassium hydroxide to the solution in Example 18.13. When the [OH - ] reaches 1.9 * 10 -6 M (as you just calculated), magnesium hydroxide begins to precipitate out of solution. As you...
-
The Income Statement columns of the work sheet of G. J. Hanson P.O. 2 Company for the fiscal year ended June 30 appear below. During the year, G. J. Hanson withdrew $52,000. Journalize the closing...
-
Determine the location using physics calculations to solve the problem. Show step by step details for how you solved the problem. I don't need an explanation explaining how to solve the problem. T By...
-
Give a brief explanation about the organization/company i.e., the products or services, number of employees, etc. Do a SWOT chart to help organize your ideas. Refer to resources in the reading for an...
-
How are organization "formal" and "informal" structures impacted in organizational change? Provide some examples. Compare and contrast Lewin's Change Model with Kotter's Change model. (Show how they...
-
Use as many directional terms as possible to describe therelationshipbetween: a. the antecubital region and the poplitealregion b. the acromial region and the mentalregion c. the gluteal region and...
-
Average rate of return-cost savings Maui Fabricators Inc. is considering an investment in equipment that will replace direct labor. The equipment has a cost of $114,000 with a $10,000 residual value...
-
Find the wattmeter reading of the circuit shown in Fig. 11.93. 1012 20 cos 4 V 4 13
-
(a) What is the focal length of a magnifying glass that gives an angular magnification of 8.0 when the image is at infinity? (b) How far must the object be from the lens?
-
Obtain the Laplace transform of f (t) in Fig. 15.28 . f(t) A 15 1 2 3 4 t
-
Find the Laplace transform of the signal in Fig. 15.26. f(t) A 25 2 4
-
Determine the Laplace transform of 3.5 cos (5t 45).
-
By the help of Conditioning Formatting (not manually!) underline the managers who impacted with the highest and the lowest sales. Post your results in Problem1 sheet
-
Galloway Inc is an over levered public company, with two classes of shares. Since the insiders (who run the company) own the voting shares, the company has the luxury of decreasing its debt ratio...
-
Zheng Corporation plans to issue new bonds to finance its expansion plans. In its efforts to price the issue, Zheng Corporation has identified a company of similar risk with an outstanding bond issue...
Pgm Golf Club Set Junior Golf Club Set For Kids Children Right Handed - ISBN: B0C5HYF45K - Free Book

Study smarter with the SolutionInn App


