An aspirin tablet contains 325 mg of acetylsalicylic acid (C 9 H 8 O 4 ). How
Question:
An aspirin tablet contains 325 mg of acetylsalicylic acid (C9H8O4). How many acetylsalicylic acid molecules does it contain?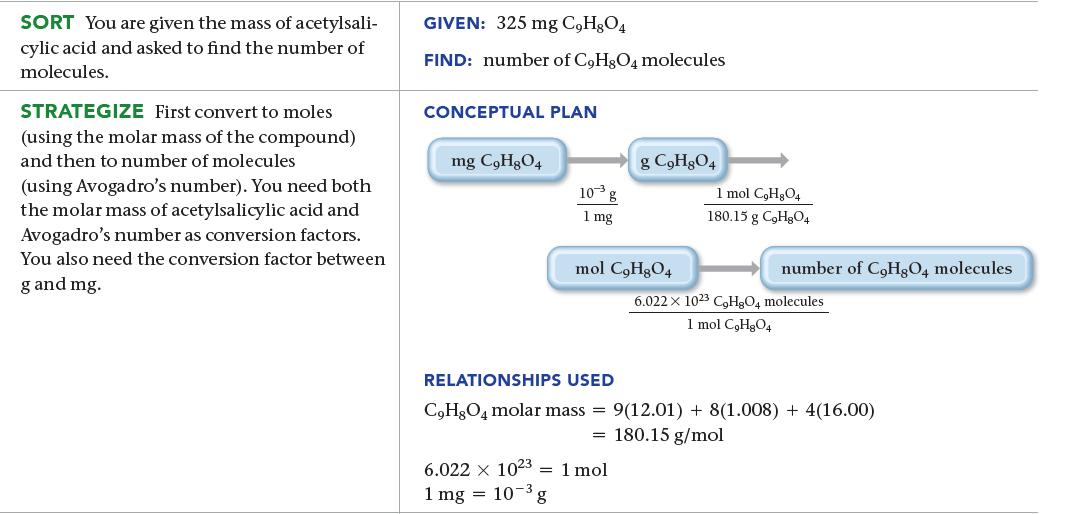
Transcribed Image Text:
SORT You are given the mass of acetylsali- cylic acid and asked to find the number of molecules. STRATEGIZE First convert to moles (using the molar mass of the compound) and then to number of molecules (using Avogadro's number). You need both the molar mass of acetylsalicylic acid and Avogadro's number as conversion factors. You also need the conversion factor between g and mg. GIVEN: 325 mg C,H,O4 FIND: number of C9H8O4 molecules CONCEPTUAL PLAN mg C₂H₂O4 10³ g 1 mg g C₂H8O4 mol C₂H₂O4 6.022 x 1023 1 mol 1 mg = 10-3, ³g 1 mol C₂H₂O4 180.15 g C₂H8O4 number of CoHgQ4 molecules 6.022 x 1023 C₂H8O4 molecules 1 mol C₂H₂O4 RELATIONSHIPS USED C₂H8O4 molar mass= 9(12.01) + 8(1.008) + 4(16.00) = 180.15 g/mol
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
325 mg CH8O4 X 10 g 1 m...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A typical aspirin tablet contains 325 mg acetylsalicylic acid (HC 9 H 7 O 4 ). Calculate the pH of a solution that is prepared by dissolving two aspirin tablets in enough water to make one cup (237...
-
Given f(x) is a polynomial function with leading term of -24x^(11), a. How many zeros will f(x) have? b. How many x-intercept s can f(x) have? c. How many turning points will f(x) have?
-
Given a rigid tank with V=800 L filled with m = 2 kg of HO at P = 0.2 MPa. Write your answers in the table below. Only answers in the table are counted. Specific volume (m/kg) - R Phase description:...
-
What is marginal cost Explain with an example.
-
In the previous problem, suppose your required return on the project is 20 percent and your pretax cost savings are $340,000 per year. Will you accept the project? What if the pretax cost savings are...
-
A Gallup poll indicated that 20% of Americans had confidence in U.S. banks. Interestingly, 58% also said that they had confidence in their main or primary bank. (data extracted from D. Jacobs,...
-
Explain the nature of creative accounting. L01
-
On November 1, 2007, Janet Morton and Kim Wong formed Pet Kingdom, Inc., to sell pets and pet supplies. Pertinent information regarding Pet Kingdom is summarized as follows: Pet Kingdom's business...
-
What form must be used by VITA/TCE volunteers when performing a thorough interview with a taxpayer?\ a. Form 13614-C, Intake/Interview and Quality Review Sheet.\ b. Form 13614-NR, Nonresident Alien...
-
What is the formula mass for a compound? Why is it useful?
-
A compound has the empirical formula CH 2 O and a formula mass of 120.10 amu. What is the molecular formula of the compound? a) CH 2 O b) C 2 H 4 O 2 c) C 3 H 6 O 3 d) C 4 H 8 O 4
-
(a) Show that X and Y are orthogonal in Rn if and only if || X + Y || = ||X - Y||. (b) Show that X + Y and X - Y are orthogonal in Rn if and only if || X || = || Y ||.
-
The 2017 financial statements of LVMH Moet Hennessey Louis Vuitton S.A. are presented in Appendix C at the end of this book. LVMH is a Paris-based holding company and one of the world's largest and...
-
Repeat Problem 10.E1, except design a packed column using 1-in. metal Pall rings. Do the calculations at the top of the column. Approximate HETP for ethanol-water is \(0.366 \mathrm{~m}\). At...
-
We are separating an ethanol-water mixture in a column operating at atmospheric pressure with a total condenser and a partial reboiler. Constant molal overflow (CMO) can be assumed, and reflux is a...
-
Corporate Social Responsibility Problem The Global Reporting Initiative (GRI) is a networkbased organization that has pioneered the development of the world's most widely used sustainability...
-
Draw a bar graph for each data set in Problems 32-35. Data set \(\mathrm{D}\) Data set A: The annual wages of employees at a small accounting firm are given in thousands of dollars. 35 25 25 16 14 1...
-
In an experiment involving P1 transduction, the cotransduction frequency was 0.53. How far apart are the two genes?
-
What types of inventory issues Starbucks might reflect upon at the end of each year? The mission of Starbucks is to inspire and nurture the human spiritone person, one cup, and one neighborhood at a...
-
For 1.25 mol of an ideal gas, P external = P =350. 10 3 Pa.The temperature is changed from 135C to 21.2C, and C V ,m = 3/2R. Calculate q, w, U, and H.
-
Suppose an adult is encased in a thermally insulating barrier so that all the heat evolved by metabolism of foodstuffs is retained by the body. What is her temperature increase after 2.5 hours?...
-
Draw bond-line structures for all constitutional isomers of C 5 H 12 ?
-
Carter Company is considering three investment opportunities with the following payback periods: Project A Project B Project C Payback period 2.7 years 6.4 years 3.8 years Use the decision rule for...
-
Q16. Nongshin Company manufactures various types of instant noodles and the November 2021 income statement for selected two models is presented: Cold Spicy Hot Super Spicy Net Sales Revenue $12,600...
-
.com/in/take Assignment/takeAssignmentMain.do?invoker=&takeAssignmentSession locator=&inprogress=false eBook Calculator Income Statement for a Manufacturing Company Two items are omitted from each of...

Study smarter with the SolutionInn App


