An element has four naturally occurring isotopes with the masses and natural abundances given here. Find the
Question:
An element has four naturally occurring isotopes with the masses and natural abundances given here. Find the atomic mass of the element and identify it.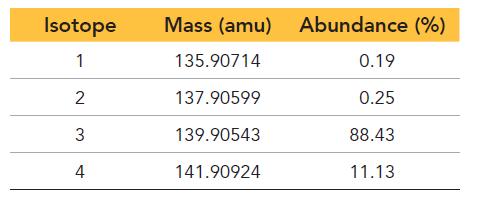
Transcribed Image Text:
Isotope 1 2 3 4 Mass (amu) 135.90714 137.90599 139.90543 141.90924 Abundance (%) 0.19 0.25 88.43 11.13
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
To find the atomic mass of an element given the masses and natural abundances of its isotopes we use ...View the full answer

Answered By

Somshukla Chakraborty
I have a teaching experience of more than 4 years by now in diverse subjects like History,Geography,Political Science,Sociology,Business Enterprise,Economics,Environmental Management etc.I teach students from classes 9-12 and undergraduate students.I boards I handle are IB,IGCSE, state boards,ICSE, CBSE.I am passionate about teaching.Full satisfaction of the students is my main goal.
I have completed my graduation and master's in history from Jadavpur University Kolkata,India in 2012 and I have completed my B.Ed from the same University in 2013. I have taught in a reputed school of Kolkata (subjects-History,Geography,Civics,Political Science) from 2014-2016.I worked as a guest lecturer of history in a college of Kolkata for 2 years teaching students of 1st ,2nd and 3rd year. I taught Ancient and Modern Indian history there.I have taught in another school in Mohali,Punjab teaching students from classes 9-12.Presently I am working as an online tutor with concept tutors,Bangalore,India(Carve Niche Pvt.Ltd.) for the last 1year and also have been appointed as an online history tutor by Course Hero(California,U.S) and Vidyalai.com(Chennai,India).
4.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The element lead (Pb) consists of four naturally occurring isotopes with atomic masses 203.97302, 205.97444, 206.97587, and 207.97663 amu. The relative abundances of these four isotopes are 1.4,...
-
An element has two naturally occurring isotopes with the following masses and abundances: Isotopic Mass (amu) Fractional Abundance 49.9472. 2.500 103 50.9440. 0.9975 What is the atomic mass of this...
-
An element has three naturally occurring isotopes with the following masses and abundances: Isotopic Mass (amu) Fractional Abundance 38.964 0.9326 39.964 1.000 104 40.962 0.0673 Calculate the atomic...
-
Given the following data about XYZ Mutual Fund on Oct. 1: Assets: Liabilities: Cash = $40,000 Accrued fees and expenses = $5,000 1,000 Shares of Stock A: Closing Price $30 2,000 Shares of Stock B:...
-
Mission Foods produces two flavors of tacos, chicken and fish, with the following characteristics: Required The total fixed costs for the company are $117,000. a. What is the anticipated level of...
-
Nationwide Discount Furniture hired Rampart Security to install an alarm in its warehouse. A fire would set off an alarm in Rampart's office, and the security company was then supposed to notify...
-
Explain the logic of the residual dividend model, the steps a firm would take to implement it, and why it is more likely to be used to establish a long-run payout target than to set the actual...
-
Jon Williams, CPA, is in the middle of the real- life soap opera, Taxing Days of Our Lives. The Cast of Characters Oneway Corporation is Williamss audit and tax client. The three directors are the...
-
Dylan (57) and Megan (56) are considering retiring in the next few years. They have some questions for you since you are their adviser. They own their own home outright and have two children, Rob...
-
You are the executive assistant to the director of sales at B-Trendz, Inc., a trendy retail store that has locations in only ten states. The company is considering branching into the online retail...
-
An element has two naturally occurring isotopes. Isotope 1 has a mass of 120.9038 amu and a relative abundance of 57.4%, and isotope 2 has a mass of 122.9042 amu. Find the atomic mass of this element...
-
Bromine has two naturally occurring isotopes (Br-79 and Br-81) and has an atomic mass of 79.904 amu. The mass of Br-81 is 80.9163 amu, and its natural abundance is 49.31%. Calculate the mass and...
-
How is depreciation handled in federal accounting and financial reporting? How does this treatment differ from that given to depreciation in municipal accounting and financial reporting?
-
screen. In Exercises 21 through 32, find the instantaneous rates of change of the given functions at the indicated points. 21. f(x) = 2x + 3, c = 2 22.) f(x) = -3x+4, c = 3 23. f(x) = x - 1, c = 1...
-
Solve . f(x)= cos(x) 2+ sin(x)
-
Hackett Produce Supply is preparing its cash budget for April. The following information is available: Estimated credit sales for April Actual credit sales for March Estimated collections in April...
-
006 10.0 points A pendulum clock was moved from a location where g = 9.8168 m/s to another location where 9 9.806 m/s. During the move, g = the length of the clock's pendulum did not change;...
-
6-3x 2 Problem 6. (a) Find L So 6-3x-2y 3 2 dz dy dx. (b) Find the limits of integration. No need to find the integral. dx dz dy. Hint: The plane in the image is given by 3x + 2y + 3z = 6. 2.0 1.5...
-
What type of mutation (transition, transversion, or frameshift) would you expect each of the following mutagens to cause? A. Nitrous acid B. 5-Bromouracil C. Proflavin
-
At the beginning of its fiscal year, Lakeside Inc. leased office space to LTT Corporation under a seven-year operating lease agreement. The contract calls for quarterly rent payments of $25,000 each....
-
Predict the products of each of the following reactions: a. b. c. d. e. f. g. h. i. 1) BH3 THF 2) H2O2, NaOH Pt
-
The rate at which two methyl radicals couple to form ethane is significantly faster than the rate at which two tert-butyl radicals couple. Offer two explanations for this observation.
-
There are only two stereo-isomers of 1, 4-dimethylcyclohexane. Draw them, and explain why only two stereo-isomers are observed.
-
Sales value and physical value alfocation
-
4. How do you calculate total manufacturing cost? a) Direct materials + Direct labor b) Direct labor + Manufacturing overhead Direct materials + Direct labor + Manufacturing overhead d) Manufacturing...
-
How does the community or people provide services from nonprofits (what challenges does the community or people face in seeking help from nonprofits)

Study smarter with the SolutionInn App


