An oxide of rhenium crystallizes with the unit cell shown here (rhenium = gray; oxygen = red).
Question:
An oxide of rhenium crystallizes with the unit cell shown here (rhenium = gray; oxygen = red). What is the formula of the oxide?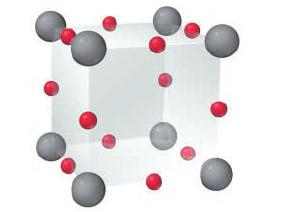
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 85% (7 reviews)
Based on the provided unit cell representation each rheniu...View the full answer

Answered By

Aysha Ali
my name is ayesha ali. i have done my matriculation in science topics with a+ . then i got admission in the field of computer science and technology in punjab college, lahore. i have passed my final examination of college with a+ also. after that, i got admission in the biggest university of pakistan which is university of the punjab. i am studying business and information technology in my university. i always stand first in my class. i am very brilliant client. my experts always appreciate my work. my projects are very popular in my university because i always complete my work with extreme devotion. i have a great knowledge about all major science topics. science topics always remain my favorite topics. i am also a home expert. i teach many clients at my home ranging from pre-school level to university level. my clients always show excellent result. i am expert in writing essays, reports, speeches, researches and all type of projects. i also have a vast knowledge about business, marketing, cost accounting and finance. i am also expert in making presentations on powerpoint and microsoft word. if you need any sort of help in any topic, please dont hesitate to consult with me. i will provide you the best work at a very reasonable price. i am quality oriented and i have 5 year experience in the following field.
matriculation in science topics; inter in computer science; bachelors in business and information technology
_embed src=http://www.clocklink.com/clocks/0018-orange.swf?timezone=usa_albany& width=200 height=200 wmode=transparent type=application/x-shockwave-flash_
4.40+
11+ Reviews
14+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
An oxide of titanium crystallizes with the unit cell shown here (titanium = gray; oxygen = red). What is the formula of the oxide?
-
Rhenium oxide crystallizes with a structure that has a primitive cubic lattice, as shown here. In the image on the left, the sizes of the ions have been reduced to show the entire unit cell. (a) How...
-
Tungsten crystallizes in the unit cell shown here. (a) What type of unit cell is this? (b) How many tungsten atoms occur per unit cell? (c) If the edge of the unit cell is 316.5 pm, what is the...
-
Different theories about early childhood inform approaches to children's learning and development. Early childhood educators draw upon a range of perspectives in their work ..." (EYLF p.12)....
-
Helvey brought suit against the Wabash County REMC (REMC) for breach of implied and express warranties. He alleged that REMC furnished electricity in excess of 135 volts to Helveys home, damaging his...
-
Describe, define, and give examples of the followingdetail the advantages and disadvantages of each: A. Mean Absolute Percentage Error. B. Mean Absolute Deviation. C. Mean Square Deviation.
-
Plaster Inc. received a $0.15-per-share cash dividend on 50,000 shares of Gestalt Corporation common stock, which Plaster Inc. carries as a long-term investment. Assuming that Plaster Inc. uses the...
-
Shank Company produces golf discs which it normally sells to retailers for $7 each. The cost of manufacturing 20,000 golf discs is: Materials ..... $ 10,000 Labor ........ 30,000 Variable overhead...
-
Allison & Co . and Bee, Inc., reported the following numbers ( in millions ) for fiscal year 2 0 1 8 . \ table [ [ , Allison & Co . , Bee, Inc. ] , [ Net income,$ 5 9 5 . 7 0 , $ 2 1 7 . 2 4
-
Consolidation related simulation example: Millennium Capital Management, Inc., (MCM) acquired a 90% interest in NextGen, Inc. MCM's Financial Manager, Matthew Steven, has prepared a draft memo to the...
-
The unit cells for cesium chloride and barium chloride are shown here. Show that the ratio of cations to anions in each unit cell corresponds to the ratio of cations to anions in the formula of each...
-
Which solid in each pair has the higher melting point and why? a. Fe(s) or CCl4(s) C. Ti(s) or Ne(s) b. KCI(s) or HCl(s) d. HO(s) or HS(s)
-
On April 1, Year 2, Maine Corporation paid $18,000 cash in advance for a one-year lease on an office building. Assume that Maine records the prepaid rent as an asset and that the books are closed on...
-
Assume the same facts as Problem 3, except both states use a three-factor formula where sales are double weighted. For the property factor, State 1 uses book value, while State 2 uses net book value....
-
Lyn Corporation has its manufacturing, distribution, and retail operations in State 1. However, sales are made to customers in States 1, 2, and 3. Sales in State 1 are made through retail stores....
-
Keep-on-Truckin Corporation (KOTC) is a manufacturer and distributor of shoes. It has established electronic data interchange (EDI) links with most of its customers. The sequence of electronic...
-
I think the waiter wrote in an extra \($25\) tip on my Sunshine Caf bill after I received and signed my credit card receipt, Mark Otter said to the restaurant manager, Brad Gladiolus. Mr. Otter, mail...
-
The following transactions occurred during January, the first month of operations for Red Corporation. Prepare journal entries and create a T-account for inventory that includes the following five...
-
Draw three-dimensional structures for the two enantiomers of the chiral compound in Problem 5.3. Problem 5.3 Which of the following compounds is chiral? a. 1-bromo-1-phenylethane b....
-
A manufacturer can sell product 1 at a profit of $20 per unit and product 2 at a profit of $40 per unit. Three units of raw material are needed to manufacture one unit of product 1, and six units of...
-
a. The stratosphere begins at 11 km above the Earths surface. At this altitude P = 22.6 kPa and T = 56.5oC. What is the mean free path of N 2 at this altitude? b. The stratosphere extends to 50 km,...
-
a. Determine the total collisional frequency for CO 2 at 1 atm and 298 K. b. At what temperature would the collisional frequency be 10% of the value determined in part (a)? For CO 2 , = 5.2 10 19 m...
-
a. A standard rotary pump is capable of producing a vacuum on the order of 10 3 Torr. What is the single-particle collisional frequency and mean free path for N 2 at this pressure and 298 K? b. A...
-
Practical Corporation is liquidated, with Neha receiving property having an adjusted basis of $60,000 and an FMV of $100,000. The property is subject to a $75,000 mortgage, which Neha assumes. Neha's...
-
6. Last year Mason Inc had a total assets turnover of 1.33 and an equity multiplier of 1.75. Its sales were $195,000 and its net income was $10,549. The CFO believes that the company could have...
-
Cover-to-Cover Company is a manufacturer of shelving for books. The company has compiled the following cost data, and wants your help in determining the cost behavior. After reviewing the data,...

Study smarter with the SolutionInn App


