At 473 K, for the elementary reaction 2 NO(g) + Cl 2 (g) A sample of NOCl
Question:
At 473 K, for the elementary reaction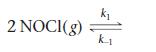
2 NO(g) + Cl2(g)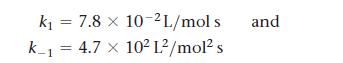
A sample of NOCl is placed in a container and heated to 473 K.
When the system comes to equilibrium, [NOCl] is found to be 0.12 mol/L. What are the concentrations of NO and Cl2?
Transcribed Image Text:
2 NOCI(g) k₁ k1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (6 reviews)
For the reaction 2NOCI 2NO Cl2 lets define the initial ...View the full answer

Answered By

Navashree Ghosh
I believe in quality work and customer satisfaction. So, I can assure you that you will get quality work from me when you hire me. Let's work together and build a long-term association.
4.90+
82+ Reviews
116+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A 0.831-g sample of SO3 is placed in a 1.00-L container and heated to 1100 K. The SO3 decomposes to SO2 and O2: At equilibrium the total pressure in the container is 1.300 atm. Find the values of Kp...
-
For the reaction below, Kp = 1.16 at 800oC. CaCO3(s) CaO(s) + CO2(g) If a 20.0- g sample of CaCO3 is put into a 10.0- L container and heated to 800oC, what percentage by mass of the CaCO3 will react...
-
At 35oC, K = 1.6 10-5 for the reaction 2NOCl(g) 2NO(g) + Cl2(g) Calculate the concentrations of all species at equilibrium for each of the following original mixtures. a. 2.0 moles of pure NOCl in...
-
Page ranks. Design a graph in which the highest-ranking page has fewer links pointing to it than some other page.
-
The Eatery is a restaurant in DeKalb, Illinois. It specializes in deluxe sandwiches in a moderate price range. Michael Raye, the manager of The Eatery, has determined that during the last 2 years the...
-
Consider Alcatel-Lucents project in Problem 6. a. What is the free cash flow to equity for this project? b. What is its NPV computed using the FTE method? How does it compare with the NPV based on...
-
What information is typically stored in a bar code or RFID tag?
-
A Sendai clothing wholesaler was preparing its sales budget for the first quarter of 20X8. Forecast sales are as follows (All values are in thousands of yen). January . 203,000 February 227,000...
-
Given the following tax structure, Taxpayer Salary Total tax Mae $ 40,500 $ 2,349 Pedro $ 53,000 ??? A. What is the minimum tax that Pedro should pay to make the tax structure vertically equitable...
-
Three different reactions involve a single reactant converting to products. Reaction A has a half-life that is independent of the initial concentration of the reactant, reaction B has a half-life...
-
The rate of decomposition of N 2 O 3 (g) to NO 2 (g) and NO(g) is followed by measuring [NO 2 ] at different times. The following data are obtained. The reaction follows a first-order rate law....
-
Suppose you wish to minimize the size of closures in a language implementation that uses a display to access nonlocal objects. Assuming a language like Pascal or Ada, in which subroutines have...
-
The WRX can travel 1 / 4 of a mile in 1 3 . 9 sec . Calculate the acceleration over this distance if assumed constant.
-
You are expected to develop two simulators mimicking the behavior and analyze the performance of iterative multiplication algorithm and add-and-shift multiplication algorithm. You are free to use any...
-
A.BRK Company, which Manufactures bags, has a Capacity of 130,000 bags per month. Currently its operating capacity is 100,000 units. The company receives a special order of 20,000 bags at $9 a bag. A...
-
Stefney Christian Date: 06/26/2023 To: From: New England Patriot Subject: Analysis of Aircraft Purchase vs. Chartering Decision I've done a thorough analysis of the decision to buy or charter a plane...
-
The Giovonis' monthly income is $9000. The have 14 remaining payments of $269 on a new car and 16 payments of $70 remaining on their living room furniture. The taxes and insurance on the house are...
-
Find the effective value of f(t) defined in Fig. 11.65. f(t) -1 0 23 4 5
-
What is the shape of the exponential distribution?
-
Calculate the depth of flow of water in a rectangular channel 10 ft wide, made of brick in cement mortar, for a discharge of 150 ft 3 /s. The slope is 0.1 percent.
-
Figure 14.21 represents the approximate shape of a natural stream channel with levees built on either side. The channel is earth with grass cover. Use n = 0.04. If the average slope is 0.000 15,...
-
A storm drainage channel in a city where heavy sudden rains occur has the shape shown in Fig. 14.20. It is made of unfinished concrete and has a slope of 0.5 percent. During normal times, the water...
-
Marietta Marine, Inc., has a traditional Section 401(k) plan. The actual deferral percentage (ADP) for all eligible non-highly compensated employees (non-HCEs) is 4%. What is the maximum ADP for the...
-
How long does a seller have to cure after shipping non-conforming goods?
-
Forward exchange contract designated as a fair value hedge of a foreign-currency-denominated accounts payable, strengthening $US On October 20, 2018, our company purchased from a company located in...

Study smarter with the SolutionInn App


