Balance the redox reactions by following the steps in the text. Rotate through the group, having each
Question:
Balance the redox reactions by following the steps in the text.
Rotate through the group, having each group member do the next step in the process and explain that step to the rest of the group.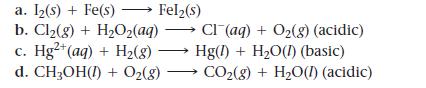
Transcribed Image Text:
a. Iz(s) + Fe(s) > Fel₂(s) b. Cl₂(g) + H₂O₂(aq) c. Hg²+ (aq) + H₂(8) d. CH3OH(1) + O₂(g) Cl(aq) + O₂(g) (acidic) Hg(1) + H₂O(1) (basic) CO₂(g) + H₂O(1) (acidic)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 87% (8 reviews)
a 12s Fes Fels 1 Assign Oxidation States Fe is oxidized and I has an oxidation state of 1 2 Write HalfReactions Oxidation HalfReaction 12s 12e Fe3 Red...View the full answer

Answered By

Wahome Michael
I am a CPA finalist and a graduate in Bachelor of commerce. I am a full time writer with 4 years experience in academic writing (essays, Thesis, dissertation and research). I am also a full time writer which assures you of my quality, deep knowledge of your task requirement and timeliness. Assign me your task and you shall have the best.
Thanks in advance
4.90+
63+ Reviews
132+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
List three specific parts of the Case Guide, Objectives and Strategy Section (See below) that you had the most difficulty understanding. Describe your current understanding of these parts. Provide...
-
THIRD AVENUE SOFTWARE HEALTH-CARE APP PROJECT This case is new for the ninth edition of Information Technology Project Management . The case provides an opportunity to apply agile and Scrum...
-
If a natural disaster, such as the 2010 drought in Russia, hits food production, use supply and demand analysis to figure out how this affects consumers and producers. Does everyone lose or are some...
-
Eggars produced 572,000 units last year. The information on the actual costs and budgeted costs at actual production of four activities follows. Required: Prepare an activity-based performance report...
-
Kendra disposed of shares of A Ltd., a qualified small business corporation (QSBC), in the current year realizing a taxable capital gain of $350,000. She has net capital losses from last year of...
-
Provide examples of how P&G may work with Kroger to jointly plan and implement a retail strategy using the six Ps.
-
Using the data set agsrs.dat, estimate the total number of acres devoted to farming for each of two domains: (a) Counties with fewer than 600 farms, and (b) Counties with 600 or more farms. Give...
-
What is the Variable MOH Spending Variance? a. $8,675 unfavorable b. $8,675 favorable c. $26,000 unfavorable d. $26,000 favorable e. None of the above I get d as an answer but the answer key says the...
-
In this chapter, you have seen that the voltage of an electrochemical cell is sensitive to the concentrations of the reactants and products in the cell. As a result, electrochemical cells can be used...
-
Which oxidizing agent will oxidize Br but not Cl? a. K 2 Cr 2 O 7 (in acid) b. KMnO 4 (in acid) c. HNO 3
-
Compare and contrast short-term memory (STM) and long-term memory (LTM) relative to storage capacity and duration of the memory.
-
Explain in details the reasons for your classifications. Classify the following processes as batch, continuous, or semibatch, and transient or steady- state. 1. A balloon is filled with air at a...
-
Question 5. A first responder drone of mass m slug is launched with a velocity vo ft/sec and constant engine force F from a level ground and moves vertically upward to discover a sense of life in a...
-
As part of the investigation of the collapse of the roof of a building. a testing laboratory is given all the available bolts that connected the steel structure at 3 different positions on the roof....
-
1) Baris Diary Co. has three product and divisions for production process of Milk, Yogurt and Cheese. Company's data show following resulst for 2014: Milk Yogurt Revenue 100.000TL 125.000TL Cheese...
-
Problem 11-4B (Algo) Prepare a statement of cash flows-indirect method (LO11-2, 11-3, 11-4, 11-5) The income statement, balance sheets, and additional information for Virtual Gaming Systems are...
-
Consider the following table displaying annual growth rates for nations X, Y, and Z, each of which entered 2011 with real per capita GDP equal to $20,000: a. Which nation most likely experienced a...
-
Why is a help desk and production support critical to system implementations? Discuss its interrelationship with the problem management and reporting system.
-
With sufficient energy, it?s possible to eject an electron from an inner atomic orbital. A higher-energy electron will then drop into the unoccupied state, emitting a photon with energy equal to the...
-
With sufficient energy, it?s possible to eject an electron from an inner atomic orbital. A higher-energy electron will then drop into the unoccupied state, emitting a photon with energy equal to the...
-
With sufficient energy, it?s possible to eject an electron from an inner atomic orbital. A higher-energy electron will then drop into the unoccupied state, emitting a photon with energy equal to the...
-
When preparing government-wide financial statements, the modified accrual based governments funds are adjusted. Please show the adjustments (in journal entry form with debits and credits) that would...
-
I need help finding the callable price and call value
-
On 31 October 2022, the owner took goods for his son as a birthday gift. The cost price of the goods was R15 000

Study smarter with the SolutionInn App


