Based on their molecular structure, pick the stronger acid from each pair of oxyacids. Explain your choice.
Question:
Based on their molecular structure, pick the stronger acid from each pair of oxyacids. Explain your choice.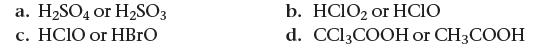
Transcribed Image Text:
a. H₂SO4 or H₂SO3 c. HCIO or HBrO b. HClO₂ or HCIO d. CC13COOH or CH3COOH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
a HSO4 more oxygen atoms bonded ...View the full answer

Answered By

Emily Grace
With over a decade of experience providing top-notch study assistance to students globally, I am dedicated to ensuring their academic success. My passion is to deliver original, high-quality assignments with fast turnaround times, always striving to exceed their expectations.
4.90+
3+ Reviews
24+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Based on their molecular structure, pick the stronger acid from each pair of binary acids. Explain your choice. a. HF and HCI b. HO or HF c. HSe or HS
-
Based on their molecular structure, pick the stronger acid from each pair of oxyacids. Explain your choice. MISSED THIS? Read Section 17.10 a. HSO4 or HSO3 c. HCIO or HBrO b. HClO or HCIO d. CCI,COOH...
-
Sam's Insurance must choose between two types of printers. Both printers meet the firm's quality standard. Printer A costs $3,500 and is expected to last 3 years with operating costs of $380 per...
-
For the transfer function below, find the constraints on K 1 and K 2 such that the function will have only two j poles. K1s + K2 T(s) = s4 + K1s + s? + K2s + 1
-
Consider the following conversation between Keri Swasey, manager of a division that produces riding lawn mowers, and her controller, Stoney Lawson, a CMA and CPA: Keri: Stoney, we have a real...
-
In the absence of limits on the behavior of large intermediaries, how might the perception of institutions being too-big-to-fail lead to increased concentration in the banking industry?
-
"The values of outstanding bonds change whenever the going rate of interest changes. In general, short-term interest rates are more volatile than long-term interest rates. Therefore, short-term bond...
-
Customers arrive at a movie theater at the advertised movie time only to find that they have to sit through several previews and prepreview ads before the movie starts. Many complain that the time...
-
What is the payback period for the following set of cash flows? (Round your answer to 2 decimal places, e.g., 32.16.) Year 0 Cash Flow $4,800 1,175 1,375 2,175 1,275 1 2 3 4 Payback period years
-
Based on molecular structure, arrange the oxyacids in order of increasing acid strength. Explain your choice. HClO3, HIO3, HBrO3
-
Based on molecular structure, arrange the binary compounds in order of increasing acid strength. Explain your choice. HTe, HI, HS, NaH
-
Using the job information in Problem 3, Construct a Gantt chart using the minimum slack per operation (MINSOP) rule to determine the job priority ranking at each machine as the jobs progress through...
-
As part of your role in the Business Analytics and Data Analytics team, you have been asked to forecast Food Retailing as part of a wider report being commissioned by the above collaboration - on...
-
You are three students who have together bought a business that makes snow. The customers consist of both large public enterprises and private individuals. The business is run all year round, but the...
-
Question 4 25 p J Mart is considering purchasing a new inventory control system featuring state-of-the-art technology. Two vendors have submitted proposals to supply J Mart with the new system. The...
-
ME 2352 Design Optimization Assignment TWO, due February 6th, 2024, 4:00 pm University of New Brunswick Department of Mechanical Engineering 1. By use of definition of linear dependency determine if:...
-
IKEA's People and Planet Positive sustainability plan, launched in 2012, aims to contribute to a better life for people and a better future for the planet. The plan outlines several sustainable goals...
-
The Grubbs' test, which is described by Algorithm 10.1, is a more statistically sophisticated procedure for detecting outliers than that of Definition 10.3. It is iterative and also takes into...
-
Burberrys competitive advantage is through its differentiation strategy. What risk should Burberry remain aware of?
-
Using Eq. (6.2), derive an expression for the focal length of a homogeneous transparent sphere of radius R. Locate its principal points. (n 1)d (n 1) R1 (6.2) R2 nRR2
-
A spherical glass bottle 20 cm in diameter with walls that are negligibly thin is filled with water. The bottle is sitting on the back seat of a car on a nice sunny day. Whats its focal length?
-
A thick glass lens of index 1.50 has radii of +23 cm and +20 cm, so that both vertices are to the left of the corresponding centers of curvature. Given that the thickness is 9.0 cm, find the focal...
-
A farmer is concerned that the price of wheat will drop by the time he is ready to sell his crop. He, therefore, enters into a futures contract on 5,000 bushels of wheat for 250 cents per bushel. The...
-
On December 1, ABC Company received $3,000 cash from a customer for 3 months of business services beginning December 1st. Prepare the journal entry to record the receipt of the 3,000 and the...
-
When Kevin started working 23 years ago, his salary was $59,349. His current salary is $159,408. When Kevin started working, the price level was 134, while the current price level is 157. What was...

Study smarter with the SolutionInn App


