Calculate G at 298 K for these reactions and predict the effect on G of lowering the
Question:
Calculate ΔG° at 298 K for these reactions and predict the effect on ΔG° of lowering the temperature.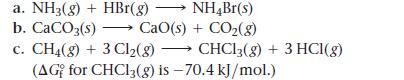
Transcribed Image Text:
a. NH3(g) + HBr(g) → NH4Br(s) b. CaCO3(s) →→→ CaO(s) + CO₂(g) c. CH4(g) + 3 Cl₂(g) → CHCl3(g) + 3 HCl(g) (AG for CHC13(g) is -70.4 kJ/mol.)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 40% (5 reviews)
a NH3g HBrg NH4Brs G H TS H 803 kJmol S 1007 JmolK G at 298 K 803 kJmol 298 K01007 kJmolK 972 kJmol ...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The conversion of natural gas, which is mostly methane, into products that contain two or more carbon atoms, such as ethane (C2H6), is a very important industrial chemical process. In principle,...
-
Carbon disulfide (CS2) is a toxic, highly flammable substance. The following thermodynamic data are available for CS2(l) and CS2(g) at 298 K: (a) Draw the Lewis structure of the molecule. What do you...
-
Consider this diagram: 1,2 90 3 3 q1 2 93 From the following, select all the correct statements. 1,2,3 a. The language recognized by this DFA is not regular. O b. This DFA describes the following...
-
Explain how each of the following illustrates one of the four principles of interaction. a. At a college tutoring co-op, students can arrange to provide tutoring in subjects they are good in (like...
-
Describe the difference between the variable overhead efficiency variance and the labor efficiency variance.
-
Show in a diagram the effect on the demand curve, the supply curve, the equilibrium price, and the equilibrium quantity of each of the following events. a. The market for newspapers in your town Case...
-
C2.4. Whymighta firm tradeat a price-to-book ratio(PIB) greaterthan l.0?
-
Required Fill in the blanks (indicated by the alphabetic letters in parentheses) in the following financial statements. Assume the company started operations January 1, 2016, and all transactions...
-
helpp! Before the year began, the following static budget was developed for the estimated sales of 100,000 Sales are sluggish and management needs to revise its budget. Use this information to...
-
Suppose we redefine the standard state as P = 2 atm. Find the new standard G f values of each substance. a. HCl(g) b. N 2 O(g) c. H(g) Explain the results in terms of the relative entropies of...
-
The standard free energy change for the hydrolysis of ATP was given in Problem 91. In a particular cell, the concentrations of ATP, ADP, and P i are 0.0031 M, 0.0014 M, and 0.0048 M, respectively....
-
One of the commonly used performance measures in the Taguchi method is Where s2 is the sample variance. In general, the higher the performance measure, the better the design. This measure is called...
-
Suppose youre applying a simulated annealing algorithm to a certain problem, where T is the parameter that measures the tendency to accept the current candidate to be the next trial solution. You...
-
Following is a partially completed balance sheet for Epsico Incorporated at December 31, 2022, together with comparative data for the year ended December 31, 2021. From the statement of cash flows...
-
Assume, further, that the acquisition was consummated on October 1, 2024, as described above. However, by the end of 2025, Ayayai was concerned that the fair values of one or both of the acquired...
-
You have been asked to prepare a brief presentation on a criminological topic or issue of interest to you. Go to the Bureau of Justice Statistics (BJS) Publications & Products Overview page (See link...
-
Sparta Fashions owns four clothing stores, where it sells a wide range of women's fashions, from casual attire to formal wear. In addition, it rents formal wear and gowns for special occasions. At...
-
The consequences of decriminalizing illegal drugs have long been debated. Some claim that legalization will lower the price of these drugs and reduce related crime and that more people will use these...
-
What will be the final value of DI after executing the following piece of code? Execute the instructions dependently one after another. CLD MOU CX,OFOH MOU AX.02874H MOU DI,01000H MOU ES, DI SUB...
-
A 50.0 mm, sharp-edge orifice is placed in a DN 100 Schedule 80 steel pipe. Compute the volume flow rate of ethylene glycol at 25C when a mercury manometer reads a 95-mm deflection.
-
An orifice meter is to be used to measure the flow rate of propyl alcohol at 25C through a PVC plastic pipe, 40 mm OD 3.0 mm wall. The expected range of flow rates is 1.0 m 3 /h to 2.5 m 3 /h....
-
A flow nozzle is to be installed in a 5-in Type K copper tube carrying linseed oil at 77F. A mercury manometer is to be used to measure the pressure difference across the nozzle when the expected...
-
You would like to have a balance of $600,000 at the end of 15 years from monthly savings of $900. If your returns are compounded monthly, what is the APR you need to meet your goal?
-
Explain the importance of covariance and correlation between assets and understanding the expected value, variance, and standard deviation of a random variable and of returns on a portfolio.
-
On August 1 , 2 0 2 3 , Mark Diamond began a tour company in the Northwest Territories called Millennium Arctic Tours. The following occurred during the first month of operations: Aug. 1 Purchased...

Study smarter with the SolutionInn App


