Calculate G rxn for the reaction: Use the following reactions and given G rxn values: CaCO3(s) CaO(s)
Question:
Calculate ΔG°rxn for the reaction:![]()
Use the following reactions and given ΔG°rxn values: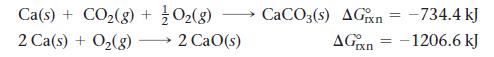
Transcribed Image Text:
CaCO3(s) CaO(s) + COz(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
To calculate the standard Gibbs free energy change AG for the reaction CaCO3s CaOs CO2g you can ...View the full answer

Answered By

Junaid ahmed
I am an English language professor with years of experience In Teaching English Language and Literature. I like to help people in the various difficult matter.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Calculation of H rxn for Part I 0 1. Assume the heat capacity of the final solution is 4.184 J K-1 g-1. Using the final mass of the solution in the calorimeter, calculate q contents from equation...
-
Proper management and leadership are key when dealing with finances in sports. Explain the difference between the two and how they will apply to the financial structure of your sports organization?
-
A builder gets a construction loan of 10 million. For simplicity, assume the loan has an interest rate at j1=6%. The loan does not require payment during the construction process. Construction will...
-
What is the formula to find total dividend and payout ratio? This is the information I have: the amount of shares the company holds and the last dividend paid. Lastly, will there be enough cash to...
-
Discuss how the globalization of markets, especially Europe after 1992, affects retail distribution.
-
Match each of the following circumstances to the corresponding element of the fraud triangle by entering the appropriate letter in the space provided. 1. Employee has significant personal debt. 2....
-
When testing a claim about a population mean or a population standard deviation, a requirement is that the sample is from a population that is normally distributed. How is this requirement different...
-
Legitron Corporation has $350 million of debt outstanding at an interest rate of 9 percent. What is the dollar value of the tax shield on that debt, just for this year, if Legitron is subject to a 35...
-
Jones Company reported pretax book income of $415,000. Included in the computation were favorable temporary differences of $51,500, unfavorable temporary differences of $20,750, and favorable...
-
Consider the reaction: 2 NO(g) + O2(g) 2 NO 2 (g) Estimate G for this reaction at each temperature and predict whether or not the reaction is spontaneous. (Assume that H and S do not change too much...
-
Determine G for the reaction: Use the following reactions with known G rxn values: FeO3(s) + 3 CO(g) 2 Fe(s) + 3 CO(g)
-
In 2011, Jed James began planting a vineyard. The costs of the land preparation, labor, rootstock, and planting were capitalized. The land preparation costs do not include any nondepreciable land...
-
BREAD Products' pretax income for 2019 is * (1 Point) BREAD Products has no Work in Process or Finished Goods inventories at the close of business on December 31, 2018. The balances of BREAD's...
-
Convert the following line of code into assembly language. A (A B)+(BA) Where A and B are both 8-bit variables Activate Windows
-
14. Create a one variable Data Table from what you just copied and pasted giving the total sales for each department, and the Largest Sale from each department. Start your Criteria range in cell A1....
-
E4.1 (LO 1), C The following independent situations require professional judgment for determining when to recognize revenue from the transactions. a. Southwest Airlines sells you an advance-purchase...
-
Spring Flings Company, a fashion retailer that specializes in colorful graphic tees, prepares a master budget on a quarterly basis. The company has assembled the following data to assist in preparing...
-
Dominion Resources, Inc., is one of the nation's largest producers of energy. Corporate headquarters are in Richmond, VA. The following is an excerpt from a recent annual report. Investments (IN...
-
At 31 December 20X9, the end of the annual reporting period, the accounts of Huron Company showed the following: a. Sales revenue for 20X9, $ 2,950,000, of which one- quarter was on credit. b....
-
Consider again the diver in Figure P8.16. Assume the diving board now has a mass of 30 kg. Find the total torque due to gravity on the diving board. Assume the mass of the board is uniformly...
-
Consider the clock in Figure 8.17. Calculate the magnitude and sign of the torque due to gravity on the hour hand of the clock at 4 o???clock. Assume the hand has a mass of 15 kg, a length of 1.5 m,...
-
A rod of length 3.8 m is hinged at one end, and a force of magnitude F = 10 N is applied at the other (Fig. P8.19).? (a) If the magnitude of the torque associated with this force is 18 N m, what is...
-
Provide a graph chart or data with sample numbers indicating Valuing Stocks and Bonds?
-
I just need help with part b. It says that the answer is not complete and some are wrong. So can you kindly fix it for me and give me the full answers as it says the answer is "not complete". Thank...
-
What is Coke's average ownership percentage in its equity method investments? Goodwill is 7000 Calculate the firm's current ratio (current assets/current liabilities). Calculate the current ratio...

Study smarter with the SolutionInn App


