Calculation of H rxn for Part I 0 1. Assume the heat capacity of the final solution is 4.184 J K-1 g-1. Using the final
Calculation of ÄH rxn for Part I 0
1. Assume the heat capacity of the final solution is 4.184 J K-1 g-1. Using the final mass of the solution in the calorimeter, calculate q contents from equation (3.9). Calculate q system from equation (3.8).
2. Calculate the moles of H2O (aq.) produced, nrxn. Be sure to identify the limiting reagent in the reaction.
3. Calculate the standard molar enthalpy change, ÄH rxn, for reaction (3.1). 0
4. CHEM 1020: Calculate the average ÄH rxn from the two runs. 0
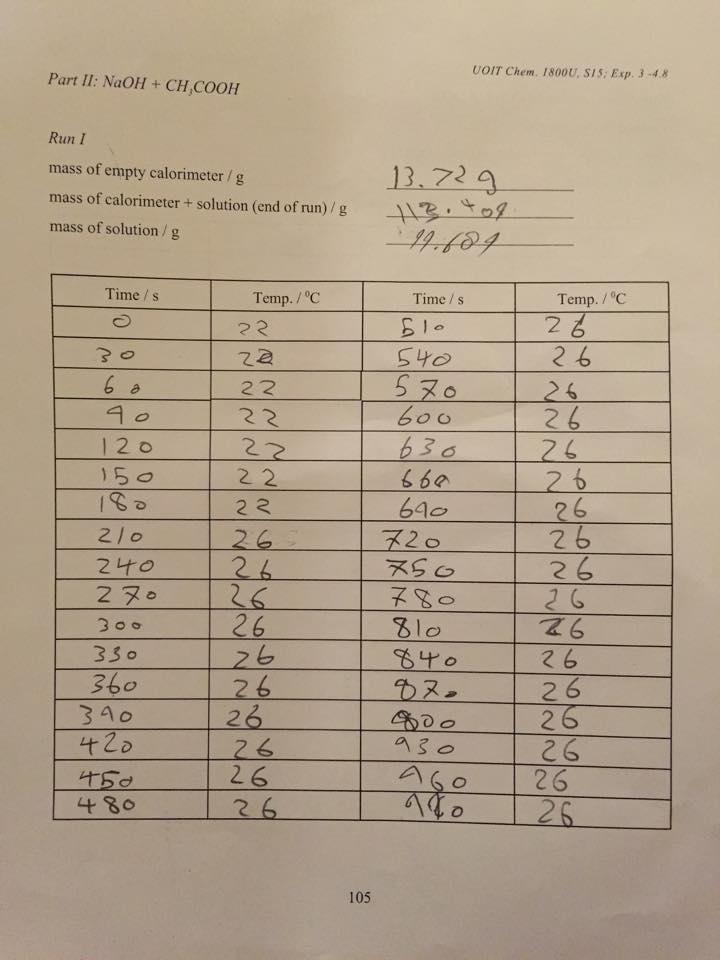
Calculation of ÄH rxn for Part II 0
1. Again, assume the heat capacity of the final solution is 4.184 J K g . Calculate qcontents and -1 -1
qsystem.
2. Calculate the number of moles of NaCH3COO(aq.) produced, nrxn. Again, identify the
limiting reagent.
3. Calculate the standard molar enthalpy change for reaction (3.2) for each of the two runs.
4. CHEM 1020: Calculate the average ÄH rxn from the two runs. 0
Questions:
1. Why should ÄH rxn for reaction (3.2) be less than ÄH rxn for reaction (3.1)?
2. Using Hess’s Law and your results from parts I and II, calculate ÄH rxn, for reaction (3.3). 0
3. Calculate the ÄH f of the OH ion given that the ÄH f (H2O) = -285.8 kJ mol . 0 - 0 -1
4. Imagine an endothermic reaction was studied (i.e. ÄH rxn > 0). If the calorimeter were not 0 perfect and qcalorimeter < 0, would the measured ÄH rxn be more or less endothermic than the 0 true ÄH rxn? 0
5. Use tabulated standard molar enthalpies of formation (from the textbook) to calculate ÄH rxn 0 for the following (unbalanced) reactions:
a. Al(s) + Fe2O3(s) ÿ Fe(s) + Al2O3(s)
b. PbO(s) + C(s) ÿ CO(g) + Pb(s)
c. CaCO3(s) ÿ CO2(g) + CaO(s)
6. The decomposition of baking soda (sodium bicarbonate) to sodium carbonate, water and carbon dioxide is endothermic. If the decomposition of 10.0 g of sodium bicarbonate requires 5.06 kJ, what is the ÄH f of sodium bicarbonate?
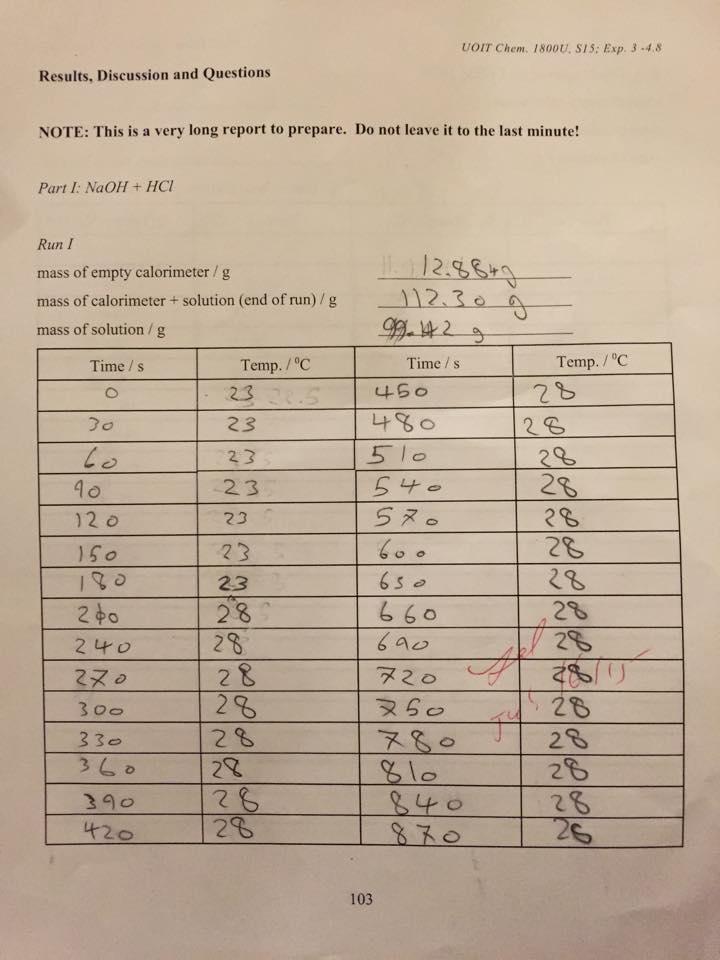
UOIT Chem. 1800U, S1S: Exp. 3 -4.8 Results, Discussion and Questions NOTE: This is a very long report to prepare. Do not leave it to the last minute! Part I: NaOH + HCI Run I mass of empty calorimeter / g mass of calorimeter + solution (end of run)/g 12.30g mass of solution/ g Time /s Temp. /C Time /s Temp. /C 23.5 450 28 23 480 28 to 23 510 28 28 28 28 540 5 10 23 12 0 23 150 23 60 . 180 23 650 28 28 660 690 200 28 240 28 28 28 0 20 300 60 28 80 810 840 28 28 28 26 330 28 360 28 28 28 390 420 103
Step by Step Solution
3.47 Rating (160 Votes )
There are 3 Steps involved in it
Step: 1
1020U 1800U W21 Ex...
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


