Calculate K p for each reaction. a. NO4(8) = 2 NO(g) b. N(g) + 3 H(g) =
Question:
Calculate Kp for each reaction.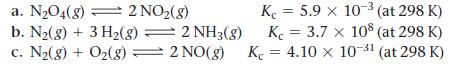
Transcribed Image Text:
a. N₂O4(8) = 2 NO₂(g) b. N₂(g) + 3 H₂(g) = 2 NH3(g) c. N₂(g) + O₂(g) — 2 NO(g) K = 5.9 x 10-³ (at 298 K) K = 3.7 x 108 (at 298 K) K = 4.10 x 10-³1 (at 298 K)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (2 reviews)
a Kp Kc RT An Anmoles of product...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
This reaction has an equilibrium constant of Kp = 2.2 * 10 6 at 298 K. Calculate Kp for each reaction and predict whether reactants or products will be favored at equilibrium. 2 COF2(g) = CO(g) +...
-
Calculate KP for the following reaction at 25C: H2(g) + I2(g) 2HI(g) G = 2.60 kJ/mol
-
Explain and critically analyze how communication could be utilized to enhance work performance and employee outcomes.
-
5. Vehicle crumple zones are designed to absorb energy during an impact by deforming to reduce transfer of energy to occupants. How much kinetic energy, in Btu, must a crumple zone absorb to fully...
-
A hospital is considering the possibility of two new purchases: new X-ray equipment and new biopsy equipment. Each project would require an investment of $750,000. The expected life for each is five...
-
A wire is bent into the shape of a quartic function \(y=a x^{4}\) and oriented in a vertical plane, with \(x\) horizontal, \(y\) vertical, and \(a\) a positive constant. A bead of mass \(m\) is...
-
Why do debt covenants often restrict the borrowing company to a certain minimum ratio of current assets to current liabilities? Why might the same covenant contain a provision that limits the annual...
-
Variance analysis, sales-mix and sales-quantity variances. Chicago Infonautics, Inc., produces handheld Windows CE???-compatible organizers. Chicago Infonautics markets three different handheld...
-
QUESTION 1 (40 marks, 48 minutes) PART A (30 marks, 36 minutes) Olivia, aged 42, is a full-time salaried employee at Healthy Pet Food ("HPF"), a private company in Pretoria, South Africa. Details of...
-
Modern Building Supply sells various building materials to retail outlets. The company has just approached Linden State Bank requesting a $300,000 loan to strengthen the Cash account and to pay...
-
Write an equilibrium expression for each chemical equation involving one or more solid or liquid reactants or products. a. CO3- (aq) + HO(1) b. 2 KCIO3(s) = 2 KCl(s) + 3 O(g) c. HF(aq) + HO(1) H3O+...
-
Calculate K c for each reaction. a. [(g) = 21(g) Kp 6.26 x 10-22 (at 298 K) b. CH4(g) + HO(g) = CO(g) + 3 H(g) c. I(g) + Cl(g) = 2 ICI(g) = Kp = 7.7 x 1024 (at 298 K) Kp = 81.9 (at 298 K) P
-
Airlines sometimes overbook flights. Suppose that for a plane with 50 seats, 55 passengers have tickets. Define the random variable Y as the number of ticketed passengers who actually show up for the...
-
During May, Darling Company incurred factory overhead costs as follows: indirect materials, $1,170; indirect labor, $2,000; utilities cost, $1,270; and factory depreciation, $5,850. Journalize the...
-
Practice 1 Let f(0) = cos(0). For each interval in the table below, determine the characteristics of f(e) Positive or negative Increasing or decreasing Concave up or concave down Let g(0) = 00
-
Given the following differential equation, dydx = sin ( x + y ) Find the following: ( a ) The substitution u = ( b ) The transformed differential equation dudx = ( c ) The implicit solution, given...
-
Consider the following type declarations TYPE Alinteger; A2 pointer to float; A3 pointer to integer; T1 structure (x: integer; } T2 structure (x: A1; next pointer to integer; } b float; } a :...
-
https://www.viddler.com/embed/82b62f65 Questions: How do companies decide where to locate their facilities? Why has just-in-time inventory control become a dominant production process used in the...
-
This exercise deals with obtaining martingales. Suppose Xt is a geometric process with drift and diffusion parameter . (a) When would the ertXt be a martingale? That is, when would the following...
-
In a certain school district, 3% of the faculty use none of their sick days in a school year. Find the probability that 5 faculty members selected at random used no sick days in a given year.
-
Determine the current flowing through an element if the charge flow is given by (a) q(t) = (3) mC (b) q(t) = (4t 2 + 20t 4) C (c) q(t) = (15e 3t 2e 18t ) nC (d) q(t) = 5t 2 (3t 3 + 4) pC (e) q(t) =...
-
How much charge is represented by these number of electrons? (a) 6.482 10 17 (b) 1.24 10 18 (c) 2.46 10 19 (d) 1.628 10 20
-
The charge entering the positive terminal of an element is q = 5 sin 4 t mC while the voltage across the element (plus to minus) is v = 3 cos 4 t V (a) Find the power delivered to the element at t...
-
If the month-end bank statement shows a balance of $75,000, outstanding checks are $54,000, a deposit of $15,000 was in transit at month end, and a check for $4,000 was erroneously charged by the...
-
SECTION A [100 MARKS] Answer ALL questions in this section. QUESTION 1 Explain the difference between financial and management accounting.
-
If Donald obtained a business loan of $270,000.00 at 5.34% compounded semi- annually, how much should he pay at the end of every 6 months to clear the loan in 25 years? Round to the nearest cent

Study smarter with the SolutionInn App


