Write an equilibrium expression for each chemical equation involving one or more solid or liquid reactants or
Question:
Write an equilibrium expression for each chemical equation involving one or more solid or liquid reactants or products.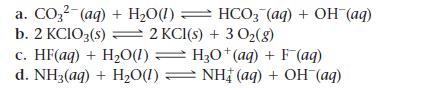
Transcribed Image Text:
a. CO3²- (aq) + H₂O(1) b. 2 KCIO3(s) = 2 KCl(s) + 3 O₂(g) c. HF(aq) + H₂O(1) H3O+ (aq) + F(aq) d. NH3(aq) + H₂O(1) ⇒ NH‡ (aq) + OH (aq) HCO3(aq) + OH (aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
a Kc c Ke ...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Write the reaction and the corresponding Kb equilibrium expression for each of the following substances (acting as bases in water). a. NH3 b. CN2 c. Pyridine, C5H5N d. Aniline, C6H5NH2
-
Hydrogen, iodine and hydrogen iodide are in equilibrium in a sealed tube at constant temperature. The equation for the reaction is: H 2 + I 2 2HI(g) H r = 96 kJ mol 1 The partial pressures of each...
-
Sodium carbonate is a diprotic base. Write a chemical equilibrium expression for each of the two successive base reactions with water.
-
As shown in Fig. 4.60, a horizontal beam is hinged to the wall at point A. The length of the beam is 1 = 2 m and it weighs W = 150 N. Point C is the center of gravity of the beam and it is...
-
Payback, Accounting Rate of Return Refer to Exercise 20-5. 1. Compute the payback period for each project. Assume that the manager of the hospital accepts only projects with a payback period of three...
-
In the HD molecule of hydrogen, one nucleus is \({ }^{1} \mathrm{H}\left(\operatorname{spin} \frac{1}{2}ight)\) and the other is a deuteron \({ }^{2} \mathrm{H}\) (spin 1). What are the possible spin...
-
Describe how audit reports may deviate from clean opinions. Explain why financial statement users should review them closely.
-
MSI is considering outsourcing the production of the handheld control module used with some of its products. The company has received a bid from Monte Legend Co. (MLC) to produce 10,000 units of the...
-
Implement your formulation in Excel and show the formula You obtain $10,000 margin loan with 5% interest to buy 100 shares of IBM $210. A year later you sold all the shares at $235. During the hold...
-
The following is Edge Sports Repair Shop's trial balance at September 30, 2014, the company's fiscal year end: Additional information: 1. Service revenue earned but not recorded at September 30,...
-
Find and fix the mistake in the equilibrium expression. PC15 (8) PC13(1) + Cl(g) Ke [PC13][C1] [PC]s]
-
Calculate K p for each reaction. a. NO4(8) = 2 NO(g) b. N(g) + 3 H(g) = 2 NH3(g) c. N(g) + O(g) 2 NO(g) K = 5.9 x 10- (at 298 K) K = 3.7 x 108 (at 298 K) K = 4.10 x 10-1 (at 298 K)
-
FIGURE 9-38 shows a block of mass 2m at rest on a horizontal, frictionless table. Attached to this block by a string that passes over a pulley is a second block, with a mass m. The initial position...
-
Assume an organization must invest $ 7 0 0 , 0 0 0 in fixed costs to produce a product that sells for $ 7 5 and requires $ 4 0 in variable costs to produce one unit. What is the organization s...
-
hotel delta marriott montreal What do you think is the value and purpose for the hotel brand choosing to make CSR an important part of their overall business strategy? What two recommendations based...
-
Consider a system consisting of a colloidal particle of radius and charge Q-+20e (e is the charge of an electron) stationary in the center of a spherical cavity of radius R=5. Its counterions have...
-
Use the Empirical Rule to determine the percentage of candies with weights between 0.7 and 0.98 gram. Hint: x=0.84.
-
A sample of 16 items provides a sample standard deviation of 9.5. Test the following hypotheses using a = .05. Ho: 0250 2 Ha > 50 a. Calculate the value of the test statistic (to 2 decimals). 27.08...
-
Consider Zt = ertXt where Xt is an exponential Wiener process: Xt = eWt (a) Calculate the expected value of the increment dZ(t). (b) Is Zt a martingale? (c) Calculate E[Zt]. How would you change the...
-
$10,000 was borrowed at 3.5% on July 17. The borrower repaid $5000 on August 12, and $2000 on September 18. What final payment is required on November 12 to fully repay the loan?
-
The current flowing past a point in a device is shown in Fig. 1.25 . Calculate the total charge through the point. i (mA) 10 2 t (ms)
-
Determine the total charge transferred over the time interval of 0 t 10 s when i( t) = 1/2 t A.
-
A total charge of 300 C flows past a given cross section of a conductor in 30 seconds. What is the value of the current?
-
E1-1 Types of businesses Indicate whether each of the following companies is primarily a service, merchandise, or manufacturing business. If you are unfamiliar with the company, you may use the...
-
The country of Lebanon just invested $334,800 to build an amusement park. The amusement park is expected to produce cash inflows of $48,300 for 9 years and a cash inflow of $63,700 in Year 10. If...
-
Which of the following would not be shown in the operating activities section of the statement of cash flows? inventory sold collections from customers payments to suppliers exchanges of assets

Study smarter with the SolutionInn App


