Calculate the change in entropy that occurs in the system when 55.0 g of water vaporizes from
Question:
Calculate the change in entropy that occurs in the system when 55.0 g of water vaporizes from a liquid to a gas at its boiling point (100.0 °C). See Table 12.7 for heats of vaporization.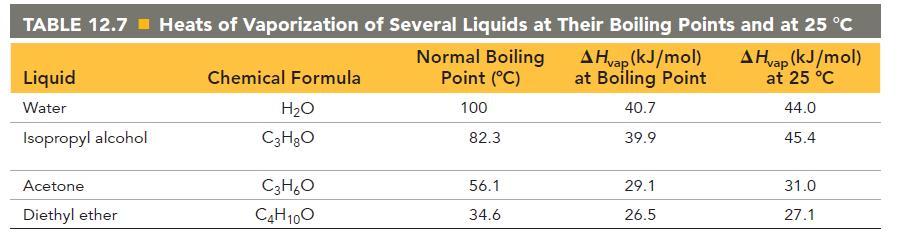
Transcribed Image Text:
TABLE 12.7 Heats of Vaporization of Several Liquids at Their Boiling Points and at 25 °C A Hvap (kJ/mol) at Boiling Point A Hvap (kJ/mol) at 25 °C 44.0 45.4 Liquid Water Isopropyl alcohol Acetone. Diethyl ether Chemical Formula H₂O C3H₂O C3H₂O C4H10O Normal Boiling Point (°C) 100 82.3 56.1 34.6 40.7 39.9 29.1 26.5 31.0 27.1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
To calculate the change ...View the full answer

Answered By

Dinesh F
I have over 3 years of professional experience as an assignment tutor, and 1 year as a tutor trainee.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Calculate the change in entropy that occurs in the system when 1.00 mole of diethyl ether (C 4 H 10 O) condenses from a gas to a liquid at its normal boiling point (34.6 C). See Table 12.7 for heats...
-
Calculate the change in entropy that occurs in the system when 1.00 mol of methanol (CH 3 OH) vaporizes from a liquid to a gas at its boiling point (64.6 C). For methanol, H vap = 35.21 kJ/mol. a)...
-
Calculate the change in entropy that occurs in the system when 45.0 g of acetone (C 3 H 6 O) freezes at its melting point (-94.8 C). See Table 12.9 for heats of fusion. TABLE 12.9 Heats of Fusion of...
-
M1 is a way to measure... a) the level of bank reserves b) a country's money supply c) the level of savings in a country d) a country's economic potential
-
Discuss how decentering is used to get an accurate translation of a questionnaire.
-
What items are included in Accumulated Other Comprehensive Income (Loss)?
-
Under valuation of closing stock in cost accounts is added while reconciling cost profit with financial profits.
-
It is the end of 2010, and, as an accountant for Newell Company, you are preparing its 2010 financial statements. On December 29, 2010, the management of Newell decided to sell one of its major...
-
GM has a preferred stock that pays a constant quarterly dividend of $3 per share. How much are you willing to pay for one share if you require a 9 percent rate of return?
-
Without doing any calculations, determine the sign of S sys for each chemical reaction. a. Mg(s) + Cl(g) MgCl(s) b. 2 HS(g) + 3 O(g) 2 HO(g) + 2 SO(g) c. 203(g) d. HCI(g) + NH3(g) NH4Cl(s) 3 O(g)
-
Calculate the change in entropy that occurs in the system when 1.00 mole of isopropyl alcohol (C 3 H 8 O) melts at its melting point (-89.5 C). See Table 12.9 for heats of fusion. TABLE 12.9 Heats of...
-
(Appendix) Hans Flims is the controller of Bavaria Labs, a manufacturer and distributor of generic prescription pharmaceuticals. He is currently preparing the annual budget and reviewing the current...
-
In Exercises 21-24, use these results from the "1-Panel-THC" test for marijuana use, which is provided by the company Drug Test Success: Among 143 subjects with positive test results, there are 24...
-
Please attached excel solution with formulars. An electronics manufacturer wants to expand its market in Europe. The demand in Europe is forecasted as: England France Spain Germany Italy Sweden 9 16...
-
Write a 1,500-2,000-word evaluation paper using the following instructions to complete this assignment. Go to the FBI Uniform Crime Reporting Program website (See link in the Class Resources). Search...
-
The y-intercept of the graph of the exponential function f(x) = 7.5(0.98)* is. Answer:
-
Using the following data definitions: bytel BYTE OFFh, 1, 2 byte2 BYTE 14h wordl WORD OFFFFh, 1, 2 word2 WORD 3 word3 SWORD 7FFFh, 8000h word4 SWORD 9000h dword1 DWORD 10h, 20h, 30h, 40h dArray DWORD...
-
Eastern Manufacturing is involved with several situations that possibly involve contingencies. Each is described below. Eastern's fiscal year ends December 31, and the 2016 financial statements are...
-
Explain why each of the following is either a private good or a public good: traffic lights, in line skates, a city park, a chicken salad sandwich, a tennis racket, national defense, a coastal...
-
Show that F{1} = 2().
-
Determine the Fourier transform of the function (x) = A cos 0 x.
-
Consider the function and first check that the exponents are unitless. Then show that the Fourier transform of E(t) is You might want to use the integral identity ,-! E(t) = Ege-i@ote-7/27 E(@) =...
-
Minden Company introduced a new product last year for which it is trying to find an optimal selling price. Marketing studies suggest that the company can increase sales by 5,000 units for each $2...
-
Prepare the adjusting journal entries and Post the adjusting journal entries to the T-accounts and adjust the trial balance. Dresser paid the interest due on the Bonds Payable on January 1. Dresser...
-
Venneman Company produces a product that requires 7 standard pounds per unit. The standard price is $11.50 per pound. If 3,900 units required 28,400 pounds, which were purchased at $10.92 per pound,...

Study smarter with the SolutionInn App


