Calculate the change in entropy that occurs in the system when 45.0 g of acetone (C 3
Question:
Calculate the change in entropy that occurs in the system when 45.0 g of acetone (C3H6O) freezes at its melting point (-94.8 °C).
See Table 12.9 for heats of fusion.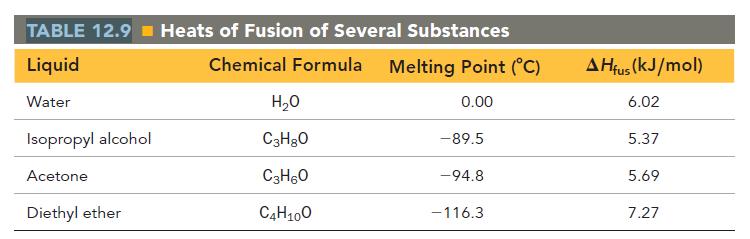
Transcribed Image Text:
TABLE 12.9 Heats of Fusion of Several Substances Liquid Chemical Formula Melting Point (°C) Water 0.00 Isopropyl alcohol Acetone Diethyl ether H₂O C3H8O C3H6O C4H10O - 89.5 -94.8 -116.3 AHfus (kJ/mol) 6.02 5.37 5.69 7.27
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 88% (9 reviews)

Answered By

Ali Khawaja
my expertise are as follows: financial accounting : - journal entries - financial statements including balance sheet, profit & loss account, cash flow statement & statement of changes in equity -consolidated statement of financial position. -ratio analysis -depreciation methods -accounting concepts -understanding and application of all international financial reporting standards (ifrs) -international accounting standards (ias) -etc business analysis : -business strategy -strategic choices -business processes -e-business -e-marketing -project management -finance -hrm financial management : -project appraisal -capital budgeting -net present value (npv) -internal rate of return (irr) -net present value(npv) -payback period -strategic position -strategic choices -information technology -project management -finance -human resource management auditing: -internal audit -external audit -substantive procedures -analytic procedures -designing and assessment of internal controls -developing the flow charts & data flow diagrams -audit reports -engagement letter -materiality economics: -micro -macro -game theory -econometric -mathematical application in economics -empirical macroeconomics -international trade -international political economy -monetary theory and policy -public economics ,business law, and all regarding commerce
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Calculate the change in entropy that occurs in the system when 1.00 mole of isopropyl alcohol (C 3 H 8 O) melts at its melting point (-89.5 C). See Table 12.9 for heats of fusion. TABLE 12.9 Heats of...
-
Calculate the change in entropy that occurs in the system when 55.0 g of water vaporizes from a liquid to a gas at its boiling point (100.0 C). See Table 12.7 for heats of vaporization. TABLE 12.7...
-
Calculate the change in entropy that occurs when 18.02 g of ice at 210.0oC is placed in 54.05 g of water at 100.0oC in a perfectly insulated vessel. Assume that the molar heat capacities for H2O(s)...
-
The largest government expenditure for Japan & the US is... a) military / defense b) transfer payments c) education / healthcare d) infrastructure
-
What are some problems created by language and the ability to comprehend the questions in collecting primary data? How can a foreign market researcher overcome these difficulties?
-
Refer to E11-12. Prepare the journal entry to record (a) the small 12 percent stock dividend and, alternatively, (b) the large 100 percent stock dividend mentioned in requirement 2 of E11-12. Refer...
-
Under absorption = actual > estimated.
-
How, if at all, should MMC report the expected loss on the large receivable in its Form 10-K financial statements? Explain.
-
Choose an Excel function/skill with Finance applications such as VBA and demonstrate and explain the topic/skill as if you are training co-workers . The content can be a function/skill you learned at...
-
Without doing any calculations, determine the sign of S sys for each chemical reaction. a. Mg(s) + Cl(g) MgCl(s) b. 2 HS(g) + 3 O(g) 2 HO(g) + 2 SO(g) c. 203(g) d. HCI(g) + NH3(g) NH4Cl(s) 3 O(g)
-
Calculate the change in entropy that occurs in the system when 1.00 mole of diethyl ether (C 4 H 10 O) condenses from a gas to a liquid at its normal boiling point (34.6 C). See Table 12.7 for heats...
-
As stated in the case, until an investigation into his company in 2006, Madoff had not registered as an investment advisor with the SEC. Please refer to the SEC website (www.sec.gov). Are all...
-
2. (40 marks) Solve for y(t) such that y" - 6y' + 15y = 2 sin(3t),
-
6. Determine output class A{ ); } public static void main(String args[]) { int x; x = 10; if (x == 10) { int y = 20; System.out.print ("x and y: y = x*2; + y); } y = 100; } System.out.print ("x and...
-
Anita and Bonita have been roommates for the past two years while they've been in graduate school. Now that they're graduating, they are each planning to move to different cities. Their one joint...
-
To what extent are business ethics assumed, or taken for granted, by people in businesses?
-
Empowered by what he has learned in this class about gender, Brady makes a friendly wager with his girfriend, Marlisa: "I bet I can guess how the men and women at the next table will behave during...
-
Indicate (by letter) the way each of the items listed below should be reported in a balance sheet at December 31, 2016. Reporting Method N. Not r C. Current liability L. Long-term liability D....
-
CRUZ, INC. Comparative Balance Sheets December 31, 2015 CRUZ, INC. Income Statement For Year Ended December 31, 2015 Required Use the indirect method to prepare the cash provided or used from...
-
With the previous problem in mind show that the inverse transform of
-
Show that if Æ(x) is real and even, its transform is real and even. Start with Eq. (11.5), use the Euler formula from Section 2.5, and assume that Æ(x) has both a real and an imaginary...
-
Given that F{(x)} = F() and F{h(x)} = H(), if and b are constants, determine F{(x) + bh(x)}.
-
Sociology
-
I am unsure how to answer question e as there are two variable changes. In each of the following, you are given two options with selected parameters. In each case, assume the risk-free rate is 6% and...
-
On January 1, Interworks paid a contractor to construct a new cell tower at a cost of $850,000. The tower had an estimated useful life of ten years and a salvage value of $100,000. Interworks...

Study smarter with the SolutionInn App


