Calculate the free energy change for this reaction at 25 C. Is the reaction spontaneous? C3H8(g) +
Question:
Calculate the free energy change for this reaction at 25 °C. Is the reaction spontaneous?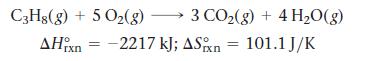
Transcribed Image Text:
C3H8(g) + 5O₂(g) 5 O₂(g) → 3 CO₂(g) + 4H₂O(g) AHin = -2217 kJ; ASixn 101.1 J/K rxn
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (6 reviews)
2247...View the full answer

Answered By

Pranav Makode
I am a bachelor students studying at professor ram meghe institute of technology and research. I have a great experience of being an expert. I have worked as an expert at helloexperts and solvelancer as a part time job. I have also worked as a doubt solver at ICAD SCHOOL OF LEARNING, which is in Amravati city. I have also worked as an Freelancer.
I have great experience of helping students, as described above. I can help any students in a most simple and understandable way. I will not give you have any chance for complaint. You will be greatfull to accept me as an expert.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Calculate the free energy change for this reaction at 25 C. Is the reaction spontaneous? 2 Ca(s) + O2(g) 2 CaO(s) 1269.8 kJ; .Sixn -364.6J/K , xn
-
Calcium oxide, CaO, is manufactured by decomposition of calcium carbonate, CaCO3, in a furnace at 500 K: CaCO3(s)?CaO(s)+CO2(g) The spontaneity of a reaction can be determined from the free energy...
-
You monitor the reaction of a protein forming a dimer and find an apparent rate constants of 6 s-1 with 1 M total protein and 10 s-1 with 5 M total protein. a. What are the forward and reverse rate...
-
How can staff review the effectiveness of their work, the services they provide and the social and cultural factors impacting on clients, groups or communities?
-
Discuss the significance of economic development to international marketing. Why is the knowledge of economic development of importance in assessing the world marketing environment? Discuss.
-
Problem Solved Company has been operating for five years as a software consulting firm. During this period, it has experienced rapid growth in Sales Revenue and in Accounts Receivable. To solve its...
-
A research service estimates that the mean annual consumption of vegetables and melons by people in the United States is at least 170 pounds per person. A random sample of 360 people in the United...
-
Selected transactions from the journal of Galaxy Inc. during its first month of operations are presented here. Instructions(a) Post the transactions to T accounts.(b) Prepare a trial balance at...
-
The Finishing Department had 5,000 incomplete units in its beginning Work-in-Process Inventory which were 100% complete as to materials and 30% complete as to conversion costs. 15,000 units were...
-
Fill in the blanks in the table. Both H and S refer to the system. T AS + AG Temperature dependent Low Temperature Spontaneous High Temperature Spontaneous Nonspontaneous Nonspontaneous
-
Calculate the change in Gibbs free energy for each of the sets of H rx n , S rx n , and T given in Problem 42. Predict whether or not each reaction is spontaneous at the temperature indicated....
-
Use the given figure for the following exercises. 1. Since \(s q\) is not an edge in Graph \(H\), Graph \(N_{1}\) cannot be a spanning tree of \(H\). a. True b. False 2. Graph \(\mathrm{N}_{2}\) is a...
-
A baseball player's slugging percentage SLG can be calculated with the following formula (which is an example of a rational function): SLG = H+2B+2x(3B)+3x(HR) AB Q Image transcription text H+2B+2x...
-
Question During 2021, Cassandra Albright, who is single, worked part-time at a doctor's office and received a W-2. She also had a cash-basis consulting practice that had the following income and...
-
Shelly Beaman (social security number 412-34-5670) Is single and resides at 540 Front Street, Ashland, NC 27898. Shelly's W-2 wages Federal withholding Social security wages Social security...
-
P14-26. Forecasting with Parsimonious Method and Estimating Share Value Using the ROPI Model Following are income statements and balance sheets for Cisco Systems. CISCO SYSTEMS Consolidated...
-
A little lesson on horseracing.An exacta wager is where you pick the horse that you think will come first, and another who will come second. A trifecta wager is where you pick 3 horses that you think...
-
On January 1, 2016, Essence Communications issued $800,000 of its 10-year, 8% bonds for $700,302. The bonds were priced to yield 10%. Interest is payable semiannually on June 30 and December 31....
-
You are maintaining a subsidiary ledger account for Police-Training Expenditures for 2013. The following columns are used: Inventory purchases are initially recorded as expenditures. Record the...
-
Runner???s gait. The amount of torque required by an external applied force (other than gravity) to move a leg forward is less than that required to move it backward if the leg is bent as shown in...
-
A wheel of mass 11 kg is pulled up a step by a horizontal rope as depicted in Figure P8.81. If the height of the step is equal to one-third the radius of the wheel, h = 1/3 R, what minimum tension is...
-
The end of a pencil of mass m and length L rests in a corner as the pencil makes an angle with the horizontal (x) direction (Fig. P8.82). If the pencil is released, it will rotate about point O with...
-
Berbice Inc. has a new project, and you were recruitment to perform their sensitivity analysis based on the estimates of done by their engineering department (there are no taxes): Pessimistic Most...
-
#3) Seven years ago, Crane Corporation issued 20-year bonds that had a $1,000 face value, paid interest annually, and had a coupon rate of 8 percent. If the market rate of interest is 4.0 percent...
-
I have a portfolio of two stocks. The weights are 60% and 40% respectively, the volatilities are both 20%, while the correlation of returns is 100%. The volatility of my portfolio is A. 4% B. 14.4%...

Study smarter with the SolutionInn App


