Calculate the change in Gibbs free energy for each of the sets of H rx n ,
Question:
Calculate the change in Gibbs free energy for each of the sets of ΔHrxn, ΔSrxn, and T given in Problem 42. Predict whether or not each reaction is spontaneous at the temperature indicated.
Problem 42
Given the values of ΔHrxn, and T, determine ΔSrxn, and predict whether or not each reaction is spontaneous.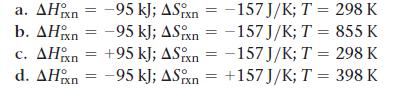
Transcribed Image Text:
a. AH n = -95 kJ; ASxn-157J/K; T = 298 K b. AH = -95 kJ; ASn = -157 J/K; T = 855 K c. AHxn = -157 J/K; T = 298 K +95 kJ; ASn = -95 kJ; ASixn -95 kJ; ASn = d. AH = +157J/K; T = 398 K
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
To calculate the change in Gibbs free energy G for each reaction we use the equation G H TS We are g...View the full answer

Answered By

OTIENO OBADO
I have a vast experience in teaching, mentoring and tutoring. I handle student concerns diligently and my academic background is undeniably aesthetic
4.30+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Calculate the change in Gibbs free energy for each of the sets of H rx n , S rxn , and T given in Problem 41. Predict whether or not each reaction is spontaneous at the temperature indicated. Problem...
-
Using data from Appendix C, calculate the change in Gibbs free energy for each of the following reactions. In each case indicate whether the reaction is spontaneous at 298 K under standard...
-
There are 2 retail companies A and B with some financial information listed in the table below. One company is a supermarket, the other is a jewellery retailer. Distinguish which is company A and...
-
when taking a lead role for supplier selection can often help Multiple select question. establish supplier quality standards. determine price. develop the engineering specifications. set conditions...
-
What are the consequences of each stage of marketing development on the potential for industrial goods within a country? For consumer goods?
-
Define long- lived assets. What are the two common categories of long- lived assets? Describe each.
-
A hat company states that the mean hat size for a male is at least 7.25. A random sample of 12 hat sizes has a mean of 7.15 and a standard deviation of 0.27. At a = 0.05, can you reject the company's...
-
Consider the following characteristics of either a JIT production system or a traditional production system. a. Products are produced in large batches. b. Large stocks of finished goods protect...
-
TipTop Flight School offers flying lessons at a small municipal airport. The school s owner and manager has been attempting to evaluate performance and control costs using a variance report that...
-
Calculate the free energy change for this reaction at 25 C. Is the reaction spontaneous? C3H8(g) + 5O(g) 5 O(g) 3 CO(g) + 4HO(g) AHin = -2217 kJ; ASixn 101.1 J/K rxn
-
Given the values of H rxn , and T, determine S rxn , and predict whether or not each reaction is spontaneous. a. AH n = -95 kJ; ASxn-157J/K; T = 298 K b. AH = -95 kJ; ASn = -157 J/K; T = 855 K c....
-
Exercise 6 Speed Dating: Attractiveness Refer to the sample data for the given exercises in Section 13-2 on page 611. Use the Wilcoxon signed-ranks test to test the claim that the matched pairs have...
-
Avery, an unmarried taxpayer, had the following income items: Salary Net income from a rental house 3 7 , 0 5 0 4 , 9 0 0 Avery has a 4 - year - old child who attends a child care center. Assume the...
-
California Lottery Let A denote the event of placing a $1 straight bet on the California Daily 4 lottery and winning. There are 10,000 different ways that you can select the four digits (with...
-
"Tamara Wiley glanced in the mirror before leaving her apartment and heading to her 8 a.m. class. She was having a bad hair day, so she had thrown on a scarf. Her quick check in the mirror told her...
-
Online Friends In a Pew Research Center survey of 1060 teens aged 13 to 17, it was found that 604 (or 57.0%) of those respondents have made new friends online. If the true rate is 50%, there is a...
-
Dr. Yong has requested that Senture Houston, an office manager at Pain Free Dental Associates, prepare a single journal entry for December 31, 2022. The bank statement for that day shows $9,500....
-
Access the FASB Accounting Standards Codification at the FASB website (www.fasb.org). Determine the spe-cific citation for accounting for each of the following items: 1. Disclosure requirements for...
-
The following selected information was taken from Sun Valley Citys general fund statement of revenues, expenditures, and changes in fund balance for the year ended December 31, 2019: Revenues:...
-
A long, non-uniform board of length 8.0 m and mass m = 12 kg is suspended by two ropes as shown in Figure P8.83. If the tensions in the ropes are mg/3 (on the left) and 2mg/3 (on the right), what is...
-
Consider again the problem of a tipping car in Example 8.6. This time, instead of applying a force F to the car, assume the car is traveling around a curve on a level road. Let the radius of...
-
A solid wood ball is rotating about an axis that passes through its center. If its angular speed is doubled, (a) by what factor does its rotational kinetic energy change? (b) By what factor does its...
-
Jenny wanted to donate to her alma mater to set up a fund for student scholarships. If she would like to fund an annual scholarship in the amount of $6,000 and her donation can earn 5% interest per...
-
You would like to have a balance of $600,000 at the end of 15 years from monthly savings of $900. If your returns are compounded monthly, what is the APR you need to meet your goal?
-
Explain the importance of covariance and correlation between assets and understanding the expected value, variance, and standard deviation of a random variable and of returns on a portfolio.

Study smarter with the SolutionInn App


