Calculate the molar solubility of magnesium fluoride (MgF 2 ) in a solution that is 0.250 M
Question:
Calculate the molar solubility of magnesium fluoride (MgF2) in a solution that is 0.250 M in NaF. For magnesium fluoride, Ksp = 5.16 * 10-11.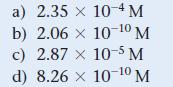
Transcribed Image Text:
a) 2.35 x 10-4 M b) 2.06 x 10-10 M c) 2.87 x 10-5 M d) 8.26 x 10-10 M
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
d ...View the full answer

Answered By

Anum Naz
Lecturer and researcher with 10+ years of experience teaching courses in both undergraduate and postgraduate levels. Supervised 17 BA theses, 07 MA theses, and 1 Ph.D. dissertations. Edited and co-authored 2 monographs on contemporary trends in political thought. Published over articles in peer-reviewed journals.
4.80+
11+ Reviews
52+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
In Exercises 125, simplify the given expression or perform the indicated operation (and simplify, if possible), whichever is appropriate. 11
-
Calculate the molar solubility of strontium sulfate, SrSO4, in 0.0015 M sodium sulfate, Na2SO4. Solve the equation exactly. See Table 17.1 for Ksp. TABLE 17.1 Solubility Product Constants, Ksp at 25C...
-
a. Calculate the molar solubility of barium fluoride, BaF2, in water at 25C. The solubility product constant for BaF2 at this temperature is 1.0 106. b. What is the molar solubility of barium...
-
Find the point in the first quadrant on the curve y = x + x 1 closest to the origin.
-
Maize Company incurs a cost of $35 per unit, of which $20 is variable; to make a product that normally sells for $58. A foreign wholesaler offers to buy 6,000 units at $30 each. Maize will incur...
-
Three laser beams have wavelengths \(\lambda_{1}=400 \mathrm{~nm}, \lambda_{2}=600 \mathrm{~nm}\), and \(\lambda_{3}=800 \mathrm{~nm}\). The power of each laser beam is \(1 \mathrm{~W}\). a. Rank in...
-
The steps involved in validating a selection battery
-
Farr Co. elects to use the percentage-of-sales basis in 2014 to record bad debt expense. It estimates that 2% of net credit sales will become uncollectible. Sales revenues are $800,000 for 2014,...
-
What can managers do to promote individual creativity
-
The magnesium and calcium ions present in seawater ([Mg 2+ ] = 0.059 M and [Ca 2+ ] = 0.011 M) can be separated by selective precipitation with KOH. What minimum [OH - ] triggers the precipitation of...
-
Calculate the molar solubility of lead(II) bromide (PbBr 2 ). For lead(II) bromide, K sp = 4.67 * 10 -6 . a) 0.00153 M b) 0.0105 M c) 0.0167 M d) 0.0211 M
-
Wisconsin Arts of Milwaukee employs five people in its Publication Department. These people lay out pages for pamphlets, brochures, and other publications for the productions. The pages are delivered...
-
31. z = x + 2xy, determine which of (I)-(II) in Figure 12.31 are cross- sections with x fixed and which are cross-sections with y fixed. (1) (11) -2 -2+
-
WHAT DOES SOCIETY EXPECT FROM ORGANIZATIONS AND MANAGERS? Introduction: TOMS Shoes has a unique idea to promote corporate social responsibility. For each pair of shoes it sells, it donates a pair to...
-
1. A car heading east turns right at a corner. The car turns at a constant speed of 20.0 m/s. After 12 s, the car completes the turn, so that it is heading due south at 20.0 m/s. Calculate the car's...
-
MTB Surfboards has a P / E of 2 0 . The discount rate for this firm is 3 0 percent. They had earnings of $ 2 , 0 0 0 , 0 0 0 and 1 0 0 , 0 0 0 shares of common stock outstanding. What should be the...
-
Question 4 (20 marks) Laboratory 4: Superposition Theorem Objectives: 1. Understand the principles of a Superposition Theorem 2. Determine the characteristics of a Superposition Theorem...
-
Consider the power system shown in Fig. 11.90. Calculate: (a) The total complex power (b) The power factor 240 V ms, 50 Hz 80-150 120t/70 600
-
How many years will it take a $700 balance to grow into $900 in an account earning 5%?
-
Design a problem to help other students better understand low-pass filters described by transfer functions. Determine the cutoff frequency of the lowpass filter described by Find the gain in dB and...
-
Find the transfer function V o V s of the circuit in Fig. 14.86 . Show that the circuit is a low-pass filter. 10 H 1F= vo 0.25 2 Vs
-
Using Fig. 14.80 , design a problem to help other students better understand the quality factor, the resonant frequency, and bandwidth of RLC circuits. For the circuits in Fig. 14.80, find the...
-
If the month-end bank statement shows a balance of $75,000, outstanding checks are $54,000, a deposit of $15,000 was in transit at month end, and a check for $4,000 was erroneously charged by the...
-
SECTION A [100 MARKS] Answer ALL questions in this section. QUESTION 1 Explain the difference between financial and management accounting.
-
If Donald obtained a business loan of $270,000.00 at 5.34% compounded semi- annually, how much should he pay at the end of every 6 months to clear the loan in 25 years? Round to the nearest cent

Study smarter with the SolutionInn App


