Calculate the vapor pressure at 25 C of a solution containing 99.5 g sucrose (C 12 H
Question:
Calculate the vapor pressure at 25 °C of a solution containing 99.5 g sucrose (C12H22O11) and 300.0 mL water.
The vapor pressure of pure water at 25 °C is 23.8 torr. Assume the density of water is 1.00 g/mL.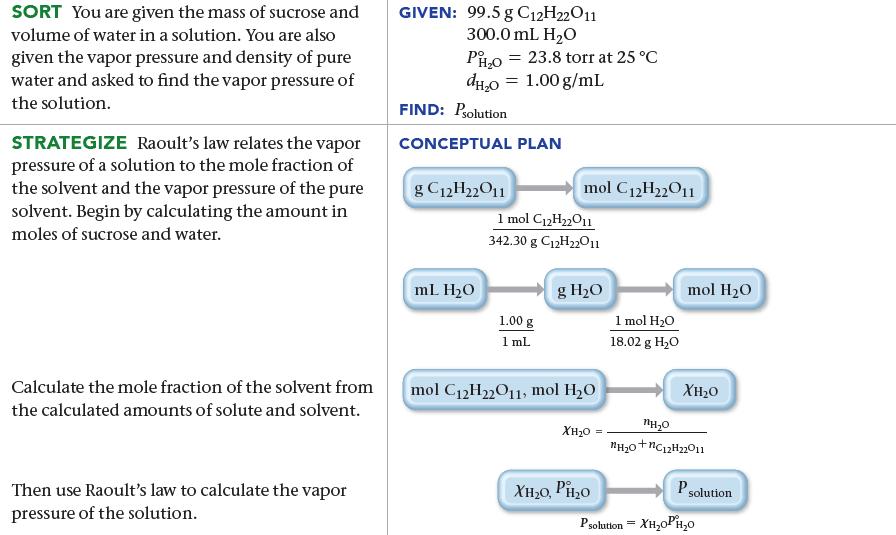
Transcribed Image Text:
SORT You are given the mass of sucrose and volume of water in a solution. You are also given the vapor pressure and density of pure water and asked to find the vapor pressure of the solution. STRATEGIZE Raoult's law relates the vapor pressure of a solution to the mole fraction of the solvent and the vapor pressure of the pure solvent. Begin by calculating the amount in moles of sucrose and water. Calculate the mole fraction of the solvent from the calculated amounts of solute and solvent. Then use Raoult's law to calculate the vapor pressure of the solution. GIVEN: 99.5 g C12H22O11 300.0 mL H₂O PH₂O dH₂0 FIND: Psolution CONCEPTUAL PLAN g C12H22011 mL H₂O = 23.8 torr at 25 °C 1.00 g/mL mol C12H22011 1 mol C₁2H₂2011 342.30 g C12H22011 1.00 g 1 mL g H₂O mol C12H22011, mol H₂O XH₂0 XH,O, PH,o 1 mol H₂O 18.02 g H₂O mol H₂O XH₂O PHO *Hạo+nC_zHzzO11 P solution Psolution = XH₂OPH₂0
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
995 g C12H22O11 X 3000 mL HO x XHO nHO nC12H2...View the full answer

Answered By

Allan Simiyu
I am an adroit Writer. I am a dedicated writer having worked as a writer for 3 years now. With this, I am sure to ace in the field by helping students break down abstract concepts into simpler ideas.
5.00+
8+ Reviews
54+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Calculate the vapor pressure at 25 C of a solution containing 165 g of the nonvolatile solute, glucose, C 6 H 12 O 6 , in 685 g H 2 O. The vapor pressure of water at 25 C is 23.8 mmHg.
-
Determine the vapor pressure at 25 C of an aqueous ethylene glycol (C 2 H 6 O 2 ) solution that is 14.8 % C 2 H 6 O 2 by mass. The vapor pressure of pure water at 25 C is 23.8 torr. a) 3.52 torr b)...
-
Calculate the vapor pressure at 35C of a solution made by dissolving 20.2 g of sucrose, C12H22O11, in 70.1 g of water. The vapor pressure of pure water at 35C is 42.2 mmHg. What is the vapor-pressure...
-
Where is the line in the sand the point where such behaviors are so destructive that you feel that the relationship needs to end?
-
Life-cycle cost reduction is best achieved during the development stage of the production life cycle. Do you agree or disagree? Explain.
-
Sorenstam Advertising Corp. was founded in January 2011. Presented below are the adjusted and unadjusted trial balances as of December 31, 2015. Instructions (a) Journalize the annual adjusting...
-
Explain accrual accounting and how it improves financial statements
-
On January 1, 2015, the ledger of Werth Company contains the following liability accounts. Accounts Payable ....... $35,000 Sales Taxes Payable ...... 5,000 Unearned Service Revenue .... 12,000...
-
Thank you so much! 3. Massey Mining Company's ore reserves are being depleted, so the firm's sales are falling. In addition, its pit is getting deeper each year, so its costs are rising. As a result,...
-
Maria sighed as she considered her new assignment. It had seemed like a great idea when Iris offered her the role, but now she wondered if she could get her arms around the complex process of getting...
-
A sodium nitrate solution is 12.5% NaNO 3 by mass and has a density of 1.02 g/mL. Calculate the molarity of the solution. a) 1.44 M b) 12.8 M c) 6.67 M d) 1.50 M
-
A solution is saturated in both nitrogen gas and potassium bromide at 75 C. When the solution is cooled to room temperature, what is most likely to happen? (a) Some nitrogen gas bubbles out of...
-
With respect to the internal control over cash, provide an example of each of the following: a. Independent checks and reviews b. Approval of transactions c. Matching documents d. Prenumbering and...
-
5.3 BEP Example Bill Braddock is considering opening a Fast 'n Clean Car Service Center. He estimates that the following costs will be incurred during his first year of operations: Rent $9,200,...
-
The following is the text for an opinion on internal control for W Company, an issuer. Some words or phrases have been replaced with numbers (e.g., [1], [2], etc.). Select from the option list...
-
Problem 14-23 (Static) Comprehensive Problem [LO14-1, LO14-2, LO14-3, LO14-5, LO14-6] Lou Barlow, a divisional manager for Sage Company, has an opportunity to manufacture and sell one of two new...
-
The farm business owes income taxes at the end of the year because income taxes are not paid until after the end of the year is true or false
-
So there were some changes in "Kieso intermediate accounting 15th edition"chapter 18 (revenue recognition). Is the test bank the same as before? If not when and what year did the update occur? Please...
-
Prove that if X1,X2, . . . are independent and identically distributed random variables having finite expectations, and if N is a stopping time for X1,X2, . . . such that E(N) -E(N)E(X) (24.58)
-
Explain what is meant by vicarious liability and when it is available?
-
What pressure is required above the water in Fig. 6.13 to cause the jet to rise to 9.50 m? The water depth is 1.50 m. 40.0 ft Air pressure h = 6.0 ft
-
What pressure is required above the water in Fig. 6.12 to cause the jet to rise to 28.0 ft? The water depth is 4.50 ft. h
-
To what height will the jet of water rise for the conditions shown in Fig. 6.40? p= 12.0 psig Jet 3.50 ft 3 in 9 in
-
Pompeii, Inc., has sales of $52,000, costs of $23,800, depreciation expense of $2,450, and interest expense of $2,200. If the tax rate is 22 percent, what is the operating cash flow, or OCF? (Do not...
-
Please provide a brief explanation as to whether the following statement is true or false. A foreign exchange trader makes very large losses that are outside the trading limits defined for her desk....
-
Amazon.com, Inc. s financial statements are presented in Appendix D. . Financial statements of Wal-Mart Stores, Inc. are presented in Appendix E. (b) What conclusions concerning the management of...

Study smarter with the SolutionInn App


