Consider the balanced equation: Complete the table showing the appropriate number of moles of reactants and products.
Question:
Consider the balanced equation:![]()
Complete the table showing the appropriate number of moles of reactants and products. If the number of moles of a reactant is provided, fill in the required amount of the other reactant, as well as the moles of each product that forms. If the number of moles of a product is provided, fill in the required amount of each reactant to make that amount of product, as well as the amount of the other product that forms.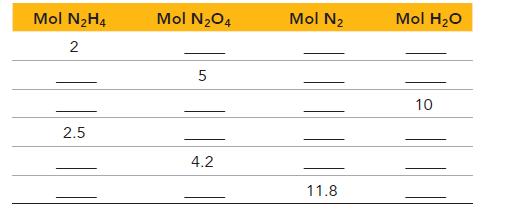
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





