Calculate how many moles of NH 3 form when each quantity of reactant completely reacts. 3 NH4(1)
Question:
Calculate how many moles of NH3 form when each quantity of reactant completely reacts.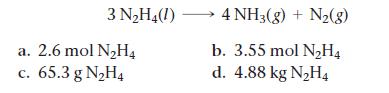
Transcribed Image Text:
3 N₂H4(1) a. 2.6 mol N₂H4 c. 65.3 g N₂H4 4 NH3(g) + N₂(8) b. 3.55 mol N₂H4 d. 4.88 kg N₂H4
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
To calculate the moles of NH3 formed when each quantity of NH completely reacts we can use the balan...View the full answer

Answered By

Stephen ouma
I have worked with different academic writing companies such as wriredom, writerbay, and Upwork. While working with these companies, I have helped thousands of students achieve their academic dreams. This is what I also intend to do here in SolutionInn
4.90+
19+ Reviews
63+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Calculate how many moles of NO 2 form when each quantity of reactant completely reacts. 2 NO5(g) a. 2.5 mol NO5 b. 6.8 mol NO5 c. 15.2 g NO5 d. 2.87 kg NO5 4 NO(g) + O(g)
-
Aluminum hydroxide reacts with sulfuric acid as follows: Which is the limiting reactant when 0.500 mol Al(OH)3 and 0.500 mol H2SO4 are allowed to react? How many moles of Al2(SO4)3 can form under...
-
A mixture of N2(g) and H2(g) reacts in a closed container to form ammonia, NH3(g). The reaction ceases before either reactant has been totally consumed. At this stage 3.0 mol N2, 3.0 mol H2, and 3.0...
-
Corning-Howell reported taxable income in 2013 of $120 million. At December 31, 2013, the reported amount of some assets and liabilities in the financial statements differed from their tax bases as...
-
In comparing accounting net income and operating cash flow, what two items do you find in net income that are not in operating cash flow? Explain what each is and why it is excluded in operating cash...
-
A 60-kg man used to have an apple every day after dinner without losing or gaining any weight. He now eats a 200-ml serving of ice cream instead of an apple and walks 20 min every day. On this new...
-
Furlong Manufacturing is considering investing in a robotics manufacturing process. Purchase and installation of the process will cost an estimated $2,900,000. This amount must be paid immediately....
-
My-Best Weight Co. offers personal weight reduction consulting services to individuals. After all the accounts have been closed on November 30, 2012, the end of the current fiscal year, the balances...
-
Question 2: CVP relation version 2 Current sales revenue is $5,000, total variable costs are $2,000, and total fixed costs are $1,000 (no data on units). a) Compute the contribution margin ratio:...
-
Q1. The structure shown consists of a S18 x 70 rolled-steel beam AB and two short members welded together and to the beam. (a) Draw the shear and bending-moment diagrams for the beam and the given...
-
Consider the balanced equation: Complete the table showing the appropriate number of moles of reactants and products. If the number of moles of a reactant is provided, fill in the required amount of...
-
Consider the unbalanced equation for the neutralization of acetic acid: Balance the equation and determine how many moles of Ba(OH) 2 are required to completely neutralize 0.461 mole of HC 2 H 3 O 2...
-
The completed financial statement columns of the work sheet for Lathrop Company arc shown below and on the next page. Instructions (a) Prepare an income statement, a retained earnings statement, and...
-
Q1)In a wheel and axle machine the diameters of the wheel and the axle are 450mm and 60mm respectively.The efficiency is 97%(0.97 per unit).When a body having a mass of 40kg is being lifted.Determine...
-
Smith & Chief Ltd. of Sydney, Australia, is a merchandising firm that is the sole distributor of a product that is increasing in popularity among Australian consumers. The company's income statements...
-
C. In lab, you measure the x & y components of a possible incompressible flow field as u = 2cxy; and where cand a are constants. v = c(a + x - y) 5. (04 pts) Short answer, what is necessary for the...
-
Year 5% 6% 4 3.546 3.465 5 7% 3.387 3.312 4.329 4.212 4.100 8% 3.993 5.076 4.917 4.767 4.623 Present Value of an Annuity of $1 at Compound Interest 9% 10% 11% 12% 13% 14% 15% 3.240 3.170 3.102 3.037...
-
2. Determine the overturning stability of the cantilever retaining wall shown. The equivalent fluid density is 5.5 kN/m, soil density is 18 kN/m, and the concrete weighs 23.5 kN/m. (5 pts) 2 m 2 m 2...
-
In fruit flies, curved wings are recessive to straight wings, and ebony body is recessive to gray body. A cross was made between true-breeding flies with curved wings and gray bodies to flies with...
-
X-1 Find the domain of the function f(x) : x 1 2 - O (-00, -1) U (-1, ) O (-00, 1) U (1, ) O -00, -1) U (-1, 1) U (1, 0) O (- 1, 1)
-
Is the equation A = U TS applicable to all processes?
-
Under what conditions is K x > K P ?
-
Rank the following compounds in order of increasing reactivity toward electrophilic aromatic substitution: Br Br- Br
-
Sunland Company uses the percentage of sales method for recording bad debts expense. For the year, cash sales are $611000 and credit sales are $2790000. Management estimates that 5% is the sales...
-
Michael will earn $148,000 in 2020. a. How much in total will be paid into the entire OASDI system (including Medicare) on behalf of this income? ___________________________ b. How much will Michael...
-
Cost = 125 000 Resdiual Value = 5000 Useful life = 10 years .. using both stright line and double declining method determine 1. Accumlated depreciation at the end of year 2 2. Book value at the...

Study smarter with the SolutionInn App


