Consider the given acid ionization constants. Identify the strongest conjugate base. Acid HNO(aq) HCHO(aq) HCIO(aq) HCN(aq) a)
Question:
Consider the given acid ionization constants. Identify the strongest conjugate base.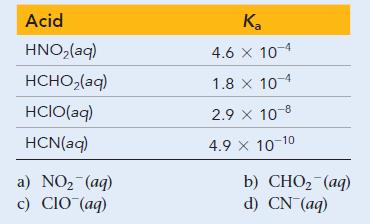
Transcribed Image Text:
Acid HNO₂(aq) HCHO₂(aq) HCIO(aq) HCN(aq) a) NO₂ (aq) c) CIO (aq) Ka 4.6 x 10-4 1.8 x 10-4 2.9 X 10 8 4.9 × 10-10 b) CHO, (aq) d) CN (aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
d...View the full answer

Answered By

Mary Boke
As an online tutor with over seven years of experience and a PhD in Education, I have had the opportunity to work with a wide range of students from diverse backgrounds. My experience in education has allowed me to develop a deep understanding of how students learn and the various approaches that can be used to facilitate their learning. I believe in creating a positive and inclusive learning environment that encourages students to ask questions and engage with the material. I work closely with my students to understand their individual learning styles, strengths, and challenges to tailor my approach accordingly. I also place a strong emphasis on building strong relationships with my students, which fosters trust and creates a supportive learning environment. Overall, my goal as an online tutor is to help students achieve their academic goals and develop a lifelong love of learning. I believe that education is a transformative experience that has the power to change lives, and I am committed to helping my students realize their full potential.
5.00+
4+ Reviews
21+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Suppose a simple random sample of size n=64 is obtained from a population that is skewed right with = 72 and = 16. (a) Describe the sampling distribution of x. (b) What is P (X>75.5)? (c) What is P...
-
Which of the following would not be acceptable for performing an insulation resistance test? a)digital multifunctional meter b)digital multimeter c)analogue insultaion resistance tester d)digital...
-
If 100 mol methanol and 100 mol acetic acid are fed to a batch reactor and allowed to come to equilibrium. How many moles of each component will be present in the product stream?
-
Given a circularly linked list L containing an even number of nodes, describe how to split L into two circularly linked lists of half the size.
-
A manufacturing plant has the theoretical capability to produce 54,000 printers per quarter but currently produces 20,250 units. The conversion cost per quarter is $2,430,000. There are 13,500...
-
Nor-Pharma AS manufactures three joint products from a joint process: Altox, Lorex and Hycol. Data regarding these products for the fiscal year ended 31 May 2022 are as follows: The joint production...
-
Describe how managers should prepare for collective bargaining, choose a negotiation team, and select a bargaining strategy. (pp. 354-358)
-
Vicki's Fabric Store shows the trial balance on page 603 as of December 31, 20-1. At the end of the year, the following adjustments need to be made: (a and b) Merchandise inventory as of December 31,...
-
The assets and Habilities of Thompson Computer Services at March 31, the end of the current year, and its revenue and expenses for the year follow. The capital of the owner was $169,300 at April 1,...
-
What is a carboxylic acid? Give an example.
-
Consider the three generic weak acids HA, HB, and HC. The images shown here represent the ionization of each acid at room temperature. Which acid has the largest Ka? (a) HA (b) HB (c) HC
-
List some advantages of a shared L2 cache among cores compared to separate dedicated L2 caches for each core.
-
WHAT DOES SOCIETY EXPECT FROM ORGANIZATIONS AND MANAGERS? Introduction: TOMS Shoes has a unique idea to promote corporate social responsibility. For each pair of shoes it sells, it donates a pair to...
-
1. A car heading east turns right at a corner. The car turns at a constant speed of 20.0 m/s. After 12 s, the car completes the turn, so that it is heading due south at 20.0 m/s. Calculate the car's...
-
MTB Surfboards has a P / E of 2 0 . The discount rate for this firm is 3 0 percent. They had earnings of $ 2 , 0 0 0 , 0 0 0 and 1 0 0 , 0 0 0 shares of common stock outstanding. What should be the...
-
Question 4 (20 marks) Laboratory 4: Superposition Theorem Objectives: 1. Understand the principles of a Superposition Theorem 2. Determine the characteristics of a Superposition Theorem...
-
2 Ursala, Inc., has a target debt-equity ratio of .65. Its WACC is 10.4 percent, and the tax rate is 23 percent. a. If the company's cost of equity is 14 percent, what is its pretax cost of debt? b....
-
Sales in a company are $196 million in 2009 and increase to $198 million in 2010. (a) Compute the percentage increase in sales using the usual formula 100 (sales2010 - Sales2009) / Sales2009...
-
What is a lobbyist in US? How did this term emerge?
-
A farsighted person can see very distant mountains with relaxed eyes while wearing +3.0D contact lenses. Prescribe spectacle lenses that will serve just as well when worn 17 mm in front of the...
-
A person who is farsighted has her near point at 100 cm and her far point is where it should normally be. Determine the prescription for a contact lens that will fix the problem. Locate her new far...
-
A 6 D myope has a far point 16.67 cm from the eye. Prescribe a spectacle lens to be worn 12 mm from the eye that will correct his vision.
-
A. What would you expect the Betas of the S&P 500 fund and the T-Bill fund to be? B. If you figure the Betas of Technology and Gold Funds to be 1.6 and 1.7, what would be the required return of an...
-
Questions are out of order photos are visible The following transactions apply to Ozark Salles for Year 1: 1. The business was started when the company received $48,000 from the issue of common...
-
Suppose your firm is considering investing in a project with the cash flows shown below, that the required rate of return on projects of this risk class is 8 percent, and that the maximum allowable...

Study smarter with the SolutionInn App


