Consider the three generic weak acids HA, HB, and HC. The images shown here represent the ionization
Question:
Consider the three generic weak acids HA, HB, and HC. The images shown here represent the ionization of each acid at room temperature. Which acid has the largest Ka?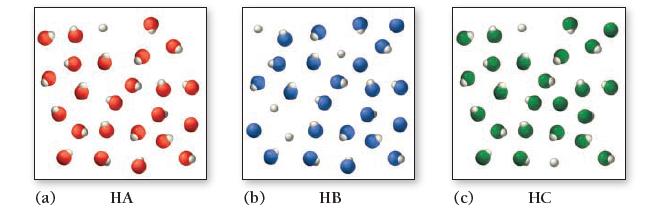
Transcribed Image Text:
(a) HA (b) HB (c) HC
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
b HB...View the full answer

Answered By

Douglas Makokha
Unlock Academic Success with Dedicated Tutoring and Expert Writing Support!
Are you ready to excel in your academics? Look no further! As a passionate tutor, I believe that dedication and hard work are the keys to achieving outstanding results. When it comes to academics, I strive to provide nothing but the best for every student I encounter.
With a relentless thirst for knowledge, I have extensively researched numerous subjects and topics, equipping myself with a treasure trove of answers to tackle any question that comes my way. With four years of invaluable experience, I have mastered the art of unraveling even the most intricate problems. Collaborating with esteemed writers has granted me exclusive access to the trade secrets utilized by the industry's top professionals.
Allow me the pleasure of assisting you with your writing assignments. I thrive on challenges and will guide you through any obstacles you may face. Together, we will unlock your academic potential and pave the way for your success.
4.90+
62+ Reviews
349+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
The diagrams here show three weak acids HA (A = X, Y, or Z) in solution. (a) Arrange the acids in order of increasing Ka. (b) Arrange the conjugate bases in increasing order of Kb. (c) Calculate the...
-
Consider the acids in Table. Which acid would be the best choice for preparing a pH = 7.00 buffer? Explain how to make 1.0 L of this buffer. Table Values of Ka for Some Common Monoprotio Acids...
-
Photons of wavelength 1.68 cm excite transitions from the rotational ground state to the first rotational excited state in a gas. Whats the rotational inertia of the gas molecules?
-
The theoretical cycle time for a product is 48 minutes per unit. The budgeted conversion costs for the manufacturing cell dedicated to the product are $4,320,000 per year. The total labor minutes...
-
Sligo Toys Ltd manufactures one type of wooden toy figure. It buys wood as its direct material for the Forming Department of its Ballinode plant. The toys are transferred to the Finishing Department,...
-
Identify mandatory, voluntary, and illegal collective bargaining issues and common economic and non-economic reasons behind bargaining. (pp. 351-354)
-
Viva Vacations operates in Tampa, Florida. The company sells time shares and also manages residential properties that it rents to vacationers. Because of the large dip in the real estate market the...
-
Please fill in all of the yellow boxes 8.00% 1,000.00 40.00 1010.00 Fixed coupon interest rate (%) Maturity value ($) Semi-annual coupon payment (C, $) Callable in 5 years at ($) Number of periods...
-
Consider the given acid ionization constants. Identify the strongest conjugate base. Acid HNO(aq) HCHO(aq) HCIO(aq) HCN(aq) a) NO (aq) c) CIO (aq) Ka 4.6 x 10-4 1.8 x 10-4 2.9 X 10 8 4.9 10-10 b)...
-
Calculate the pH of each solution at 25 C and indicate whether the solution is acidic or basic. (a) [H3O+] 1.8 x 10-4 M = (b) [OH-] = 1.3 x 10-2 M
-
Use the Repository feature to view the entry for the central process.
-
Thinking about Nike's corporate practices, discuss your approach to starting a company that outsourced labor in order to reduce manufacturing costs. What decisions would you make to combine...
-
Owen Properties recently purchased a building in a community that is eligible for participation in the National Flood Insurance Program (NFIP). Under the regular program of the NFIP, the maximum...
-
CASE 7.2 Oracle Corporation: Share-Based Compensation Effects/Statement of Shareholders' Equity A sales-based ranking of software companies provided by Yahoo! Finance on November 5, Year 8, places...
-
A manufacturer of ovens sells them for $1,450 each. The variable costs are $800 per unit. The manufacturer's factory has annual fixed costs of $1,735,000. a. Given the expected sales volume of 3,100...
-
1.1 Explain the vitality of a strategy on businesses like Dell. (15) 1.2 Critically discuss the underlying objectives Dell should follow when formulating its business strategy. (20) 1.3 Discuss the...
-
Consider the regression model Yi = 0 + 1X1i + 2X2i + ui. Use Approach #2 from Section 7.3 to transform the regression so that you can use a t-statistic to test (a) 1 = 2; (b) 1 + a2 = 0, where a is a...
-
It is possible to investigate the thermo chemical properties of hydrocarbons with molecular modeling methods. (a) Use electronic structure software to predict cHo values for the alkanes methane...
-
The vision of a hyperope is corrected with a +9D spectacle lens worn 12 mm from the cornea. Determine the appropriate power of a replacement contact lens.
-
We wish to correct the vision of a 7D my ope, whose both eyes are the same, with spectacles worn 15 mm from the eye. Determine the appropriate power.
-
An optometrist finds that a farsighted person has a near point at 125 cm. What power will be required for contact lenses if they are effectively to move that point inward to a more workable distance...
-
5. The cost of retained earnings the required rate of If a firm cannot invest retained earnings to earn a rate of return return on retained earnings, it should return those funds to its st less than...
-
How much will be the loss from bad debts under new credit terms of 3/10 net, if the cost of capital is 15% and the unpaid accounts are written off after 60 days?
-
You need to accumulate $10,000. To do so, you plan to make deposits of $1,100 per year, with the first payment being made a year from today, in a bank account that pays 7 percent annual interest....

Study smarter with the SolutionInn App


