Consider the phase diagram shown here. Identify the states present at points a through g. Pressure (not
Question:
Consider the phase diagram shown here. Identify the states present at points a through g.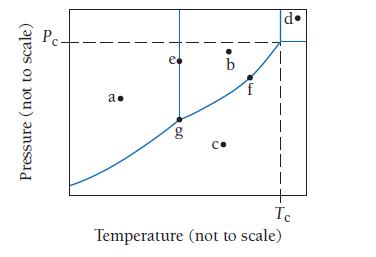
Transcribed Image Text:
Pressure (not to scale) Pc- a. e 0Q g b G 14 •Pl I Ic Temperature (not to scale)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
a Solid b Liquid ...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Explain why companies are currently turning to private credit funds to obtain debt financing, why private equity-owned companies in particular are searching for private credit financing, and explain...
-
A particular liquid crystalline substance has the phase diagram shown in the figure. By analogy with the phase diagram for a nonliquid crystalline substance, identify the phase present in each area. T
-
5. Consider the phase diagram for iodine and answer each question. a. What is the normal boiling point for iodine? b. What is the melting point for iodine at 1 atm? c. What state is present at room...
-
Given the definition of the radian angle: S l Where [s] = m, [4] = m What are the dimensions of angles? A. No way to know B. meters, m C. 1 (or no units/dimensions) D. seconds, S E. kilograms, kg
-
Iverson owned Iverson Motor Company, an enterprise engaged in the repair as well as the sale of Oldsmobile, Rambler, and International Harvester Scout automobiles. Forty percent of the businesss...
-
Prove that in the univariate case, with \(\alpha=2\), the disco decomposition is the ANOVA decomposition of variance. Start by finding an identity between SST and \(S_{2}\).
-
Discuss the possible challenges associated with performing price differentiation in practice.
-
Bill Anderson buys an automobile every 2 years at follows: initially he makes a down payment of $600C on a $15,000 car. The balance is paid in 24 equal monthly payments with annual interest at 12%....
-
Using the following data for Hayes, Inc., compute its return on assets ratio (percentage, rounded to tenths place). Hayes, Inc. Net Income 2017 $ 123,000 Total Assets 12/31/17 2,243,000 Total Assets...
-
In 2012, George wright started the Old Oregon Wood Store to man-ufacture Old Oregon tables. Each table is carefully constructed by hand using the highest- quality oak. Old Oregon tables can support...
-
Consider the phase diagram for iodine shown here. a. What is the normal boiling point for iodine? b. What is the melting point for iodine at 1 atm? c. What state is present at room temperature and...
-
How much heat (in kJ) is evolved in converting 1.00 mol of steam at 145 C to ice at -50 C? The heat capacity of steam is 2.01 J/g C, and that of ice is 2.09 J/g C.
-
Europa-Dyonisos SA produces wine. The company expects to produce 1.5 million two-litre bottles of Chablis in 2022. Europa-Dyonisos purchases empty glass bottles from an outside supplier. Its target...
-
Problem 1 Using the same Fourier-Method approach as used in lecture, consider a beam loaded as shown below. 290 -q. Cos 280 x Shane land V-280 Distributed load w = =-80 . Cos[X] a. What are the...
-
Think about a Floor Warden training program for that company - and write me another email (attached here as a Word document) as if I were the leader of your organization to tell me about the...
-
A particle travels around the curve shown, following ? = ? 0 . 2 ? ? , ?with ? ( ? ) = 0 . 5 ? 2 rad. At the moment ? = ? , ?determine the speed and acceleration of the particle. ? = , ? ? ? = , ? ?...
-
7. Chicago Corp. obtained the following information from the Raw Materials Inventory account and purchasing records for the first quarter of the current year: Beginning Raw Materials Ending Raw...
-
Suppose that i t =6% (n=1), and that future short term interest rates (n=1) for the next 3 years (starting next year) are expected to be: 4%, 2%, 2%. Suppose that the liquidity premium is zero for...
-
Provide an equation for the preparation of the following alkene from an alkyl halide. Do you anticipate problems with formation of other elimination or substitution reactions? Explain.
-
A bar of a steel alloy that exhibits the stress-strain behavior shown in Figure 6.22 is subjected to a tensile load; the specimen is 375 mm (14.8 in.) long and has a square cross section 5.5 mm (0.22...
-
In general, the high-temperature limit for the rotational partition function is appropriate for almost all molecules at temperatures above their boiling point. Hydrogen is an exception to this...
-
When 4 He is cooled below 2.17 K it becomes a superfluid with unique properties such as a viscosity approaching zero. One way to learn about the superfluid environment is to measure the...
-
Calculate the vibrational partition function for H 35 Cl ( = 2990 cm 1 ) at 300 and 3000. K. What fraction of molecules will be in the ground vibrational state at these temperatures?
-
You have been hired by Internal Business Machines Corporation (IBM) in their capital budgeting division. Your first assignment is to determine the free cash flows and NPV of a proposed new type of...
-
Burger King recently launched Real Meals in select markets to deliver an important message about mental health. Real Meals come in five varieties, including a Pissed Meal (for when youre mad) and a...
-
If banks suffer loan losses in excess of their loan-loss-reserves, their Capital Adequacy is unaffected. Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an...

Study smarter with the SolutionInn App


