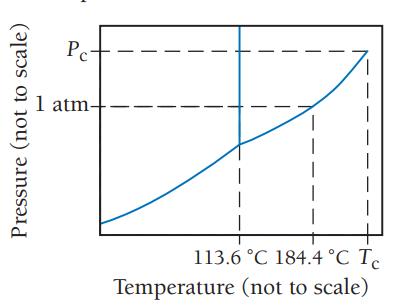
Consider the phase diagram for iodine shown here. a. What is the normal boiling point for iodine?
Question:
Consider the phase diagram for iodine shown here.
a. What is the normal boiling point for iodine?
b. What is the melting point for iodine at 1 atm?
c. What state is present at room temperature and normal atmospheric pressure?
d. What state is present at 186 °C and 1.0 atm?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 87% (8 reviews)
a Iodine normally boils at 1844C This is the temper...View the full answer

Answered By

Nazrin Ziad
I am a post graduate in Zoology with specialization in Entomology.I also have a Bachelor degree in Education.I posess more than 10 years of teaching as well as tutoring experience.I have done a project on histopathological analysis on alcohol treated liver of Albino Mice.
I can deal with every field under Biology from basic to advanced level.I can also guide you for your project works related to biological subjects other than tutoring.You can also seek my help for cracking competitive exams with biology as one of the subjects.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
In Problems 1956, solve each system of equations. If the system has no solution, state that it is inconsistent. For Problems 1930, graph the lines of the system. - - -2= = 1 -x + 2y - 3z = -4 3x-2y -...
-
Find the derivative of the function: f(x) = -4e-11x
-
Use powers of adjacency matrices to determine the number of paths of the specified length between the given vertices. Exercise 55, length 2, v 1 to v 3 Data From Exercise 55 0 1 0 0
-
A physical pendulum of mass m = 3 . 3 6 kg is comprised of an odd shape that has a centre - of - mass a distance of d = 0 . 5 5 5 m from the pivot point. The pendulum is displaced from equilibrium to...
-
On February 10, Mrs. Sunderhaus purchased a diamond ring from Perel & Lowenstein for $6,990. She was told by the companys salesperson that the ring was worth its purchase price, and she also received...
-
The \(F\)-ratio reported by anova (...) for treatment effects is not the ratio shown above. At least one is misleading for this design. Which one? Explain your reasoning.
-
What are the key barriers to youth leadership and equitable youth- adult partnerships in education, according to the research?
-
The new president of the Wernecke Company was stumped. Why had profits gone down? He had directed the sales department to push the product with the highest contribution margin , and the sales...
-
Balance sheet 2010 2011 2010 2011 Cash 4,600 3,800 Accounts payable 16,200 17,100 Accounts receivable 10,200 9,700 Long-term debt 36,000 33,400 Inventory 18,900 20,300 Common stock 14,000 20,000 Net...
-
Change Purse Inc. is a small business that is planned to be located in a small Nova Scotia town. The town was incorporated in 1889 and, like many communities in Nova Scotia, it prides itself on being...
-
Argon has a normal boiling point of 87.2 K and a melting point (at 1 atm) of 84.1 K. Its critical temperature is 150.8 K, and its critical pressure is 48.3 atm. It has a triple point at 83.7 K and...
-
Consider the phase diagram shown here. Identify the states present at points a through g. Pressure (not to scale) Pc- a. e 0Q g b G 14 Pl I Ic Temperature (not to scale)
-
International Microcircuits, Inc. Megan Bedding, vice-president of sales for International Microcircuits, Inc. (IM), was delighted when IM was one of the few firms invited to enter a bid to supply a...
-
You are facing a complex decision with several courses of possible action and probabilities associated with them. The current decision tree, based on the best possible estimates of probabilities and...
-
1. In what ways has Marriot proven an industry leader in the context of entrepreneurship in the hospitality industry. 2. What are the author's metrics of measuring entrepreneurial activity, and do...
-
Suppose you want to model the relationship between the interest rate, the economic growth rate and the inflation rate. what would be first model to fit explain.
-
Question 1 [40 marks] (a) Table 1 present experimental data related to the absorbance of two compounds over a range of concentration, in a UV-Vis cell with path length I = 1.0 cm. From this table:...
-
i. The following table presents data on wholesale gas prices for the major capital cities in the Eastern-half of Australia, from 2011-12 to 2022-23. Use this data to construct a single, time-series...
-
Combine the reaction in eq. 3.53 with a nucleophilic substitution to devise a. A two-step synthesis of b. A four-step synthesis of CH3C¡CCH2CH3 from acetylene and appropriate alkyl halides....
-
An investor sells a European call on a share for $4. The stock price is $47 and the strike price is $50. Under what circumstances does the investor make a profit? Under what circumstances will the...
-
Calculate the rotational partition function for oxygen (B = 1.44 cm 1 ) at its boiling point, 90.2 K, using the high-temperature approximation and by discrete summation. Why should only odd values of...
-
In microwave spectroscopy a traditional unit for the rotational constant is the Mc or mega cycle equal to 10 6 s 1 . For 14 N 14 N 16 O the rotational constant is 12,561.66 Mc. a. Convert the above...
-
a. Calculate the percent population of the first 10 rotational energy levels for HBr (B = 8.46 cm 1 ) at 298 K. b. Repeat this calculation for HF assuming that the bond length of this molecule is...
-
What is the difference between management's goals and the firm's goals? How can the two be in conflict? Provide examples of real world examples of when management and the owners of a company have...
-
Q) A stock price is currently $90. Over each of the next two 6-month periods, it is expected to go up by 10% or down by 10%. The risk-free interest rate is 8% per annum with continuous compounding....
-
A couple who borrow $80,000 for 30 years at 7.2%, compounded monthly, must make monthly payments of $543.03. (Round your answers to the nearest cent.) (a) Find their unpaid balance after 1 year. $...

Study smarter with the SolutionInn App


