Consider the titration curves (labeled a and b) for two weak acids, both titrated with 0.100 M
Question:
Consider the titration curves (labeled a and b) for two weak acids, both titrated with 0.100 M NaOH.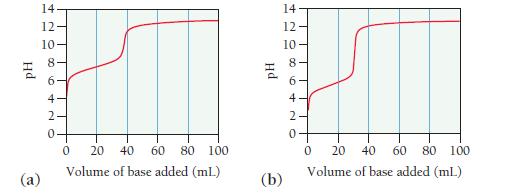
i. Which acid solution is more concentrated?
ii. Which acid has the larger Ka?
Transcribed Image Text:
Hd @ 14 12- 10 08 6 4- 420 20 40 60 80 100 Volume of base added (ml.) 0 Hd (b) 14 12 10 0 20 40 60 80 100 Volume of base added (mL) 0
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (7 reviews)
i...View the full answer

Answered By

Pushpinder Singh
Currently, I am PhD scholar with Indian Statistical problem, working in applied statistics and real life data problems. I have done several projects in Statistics especially Time Series data analysis, Regression Techniques.
I am Master in Statistics from Indian Institute of Technology, Kanpur.
I have been teaching students for various University entrance exams and passing grades in Graduation and Post-Graduation.I have expertise in solving problems in Statistics for more than 2 years now.I am a subject expert in Statistics with Assignmentpedia.com.
4.40+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
When a company issues shares of stocks it is a. Adding to the company's debts. b. Investors in the stocks are extending loans to the company. c. Issuing shares of ownership in the company. d. It is...
-
Two 25.0-mL samples of unknown monoprotic weak acids, A and B, are titrated with 0.100 M NaOH solutions. The titration curve for each acid is shown below. Which of the two weak acid solutions is more...
-
Please write detailed roadmap/solution for all questions below. 1) An output of nmap search is shown below, a) Type the required terminal command and required parameters to obtain the shown output....
-
On September 1, 2025, Swifty Corporation acquired Windsor Enterprises for a cash payment of $800,000. At the time of purchase, Windsor's balance sheet showed assets of $570,000, liabilities of...
-
Sieber has prepared the following list of statements about decision making and incremental analysis. 1. The first step in managements decision-making process is, Determine and evaluate possible...
-
Generate a table analogous to Table 15.2 for power equal to 0.80, with a = .01, two-tailed.
-
P 22-7 Journal entriesVoluntary health and welfare organization The Good Grubb Food for the Hungry Institute is a nongovernmental not-for-profit organization that provides free meals for the...
-
Wonderful! Not only did our salespeople do a good job in meeting the sales budget this year, but our production people did a good job in controlling costs as well, said Kim Clark, president of...
-
Myers Company uses a flexible budget for manufacturing overhead based on direct labor hours. Variable manufacturing overhead costs per direct labor hour are as follows. Indirect labor $1.10 Indirect...
-
A 25.0-mL sample of 0.125 M pyridine is titrated with 0.100 M HCl. Calculate the pH at each volume of added acid: 0 mL, 10 mL, 20 mL, equivalence point, one-half equivalence point, 40 mL, 50 mL....
-
Consider the titration of a 25.0-mL sample of 0.175 M CH 3 NH 2 with 0.150 M HBr. Determine each quantity. a. The initial pH b. The volume of added acid required to reach the equivalence point c. The...
-
Why do you think it took so long for LuLu's desserts to launch its export business?
-
Pacifico Company, a U.S.-based importer of beer and wine, purchased 1,200 cases of Oktoberfest-style beer from a German supplier for 264,000 euros. Relevant U.S. dollar exchange rates for the euro...
-
Finding Confidence Intervals. In Exercises 9-16, assume that each sample is a simple random sample obtained from a population with a normal distribution. Body Temperature Data Set 5 "Body...
-
19 Part 2 of 2 1.25 points Skipped Required information Problem 6-4A & 6-5A (Algo) [The following information applies to the questions displayed below.] Gerald Utsey earned $48,400 in 2021 for a...
-
Describe equilibrium constants with words and equations. is the ratio of the concentrations of products to the concentration of reactants present in a reaction mixture when chemical equilibrium is...
-
Pronghorn Inc. acquired 20% of the outstanding common shares of Gregson Inc. on December 31, 2019. The purchase price was $1,133,000 for 51,500 shares, and is equal to 20% of Gregson's carrying...
-
Maize Water is considering introducing a water filtration device for its 20-ounce water bottles. Market research indicates that 1,000,000 units can be sold if the price is no more than $3. If Maize...
-
A city maintains a solid waste landfill that was 12 percent filled at the end of Year 1 and 26 percent filled at the end of Year 2. During those periods, the government estimated that total closure...
-
A Michelson Interferometer is illuminated with monochromatic light. One of its mirrors is then moved 2.35 10 -5 m, and it is observed that 92 fringe-pairs, bright and dark, pass by in the process....
-
Examining photos of Newtons rings we observe that fringes at large values of m seem to be nearly equally spaced. To see that analytically, show that When m is large, the spacings between consecutive...
-
When dust gets between the glass elements of a Newtons ring setup, it can cause an unknown shift in the film thickness Îd, and a corresponding change in the fringe pattern. The path difference...
-
1,600 Balance Sheet The following is a list (in random order) of KIP International Products Company's December 31, 2019, balance sheet accounts: Additional Paid-In Capital on Preferred Stock $2,000...
-
Question 3 4 pts 9 x + 3 x 9 if x 0 Find a) lim f(x), b) lim, f(x), C), lim , f(x) if they exist. 3 Edit View Insert Format Tools Table : 12pt M Paragraph B IV A2 Tv
-
Mr. Geoffrey Guo had a variety of transactions during the 2019 year. Determine the total taxable capital gains included in Mr. Guo's division B income. The transactions included: 1. On January 1,...

Study smarter with the SolutionInn App


