Consider the zinc blende structure in Figure 13.16. What type of structure would result if the remaining
Question:
Consider the zinc blende structure in Figure 13.16. What type of structure would result if the remaining tetrahedral sites in the unit cell were also filled with cations?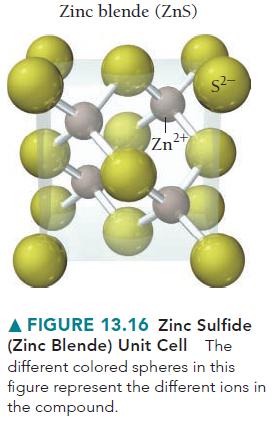
Transcribed Image Text:
Zinc blende (ZnS) Zn²+ S²- A FIGURE 13.16 Zinc Sulfide (Zinc Blende) Unit Cell The different colored spheres in this figure represent the different ions in the compound.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
If the remaining tetrahedral sites in the zinc blende structure were also filled with cations the re...View the full answer

Answered By

Swati gupta
Professionally I am going to become a Cost and Management Accountant very soon. I am a fresher in the area of tutoring but I am always admired and praised by my friends for teaching them. They always ask me to start giving tutoring services professionally. With the help of this platform, I am looking forward to give my best.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
what does the following print #include 2 class Parent{ public: Parent () { cout
-
Show that the unit cells for CaF 2 and TiO 2 in Figure 12-50 are consistent with their formulas. Figure 12-50 = Zn+ (a) Unit cell of ZnS, the zinc blende structure = $- = Ti4+ = Ca+ = 0- (c) Unit...
-
Cadmium telluride, CdTe, takes the zinc blende structure (Figure 12.26) with a unit cell edge length of 6.49 . There are four cadmium atoms and four tellurium atoms per unit cell. How many of each...
-
Happy Hands Company has net profit margin 5.51%, total assets turnover 0.63, and equity multiplier 2.77. What is its return on shareholder equity (ROE) using DuPont analysis method
-
Max E. Pass, Jr., and his wife, Martha N. Pass, departed in an aircraft owned and operated by Mr. Pass from Plant City, Florida, bound for Clarksville, Tennessee. Somewhere over Alabama the couple...
-
The gravity model is often used not only to explain trade between two countries but also to investigate the reasons why they dont. Illustrate this anomaly with suitable examples and reasons.
-
Discuss at least three methods that the ministry should use to implement your support-building recommendation. Tekram is an island nation in the South Pacific. The climate is tropical and the nation...
-
Record the following transactions in General Ledger accounts of the General Fund of Fergieville. 1. Incurred salaries of $300,000, $280,000 of which was paid. 2. A long-term note ($400,000 face...
-
just double checking my answer: Gallonte Inc. began operations in April of this year. It makes all sales on account, subject to the following collection pattern: 30% are collected in the month of...
-
A factory produces office chairs. According to the past data, the weekly demand has the following probability distribution. The selling price per chair is $120. In addition, the historical data...
-
Classify each of the following as a component of a silicate ceramic, an oxide ceramic, or a nonoxide ceramic. a. B 4 C b. Mg 2 SiO 4 c. MoSi 2
-
Consider the rock salt structure in Figure 13.15. What type of structure would result if all the anions were somehow removed, leaving only cations? Sodium chloride (NaCl) CI Na+ A FIGURE 13.15 Sodium...
-
Air France reports the following balance sheet for the year ended March 31, 2007. Required: A. In what order are assets listed on the balance sheet? B. Comment on other differences (IFRS relative to...
-
Teds Taxi Company (TTC) is considering the purchase of four new taxicabs. Various information about the proposed investment follows: Required: Help TTC evaluate this project by calculating each of...
-
Dayton Corp has \($2\) million to invest in new projects. The companys managers have presented a number of possible options that the board must prioritize. Information about the projects follows:...
-
Glowbright Company makes three types of long-burning scented candles. The models vary in terms of size and type of materials (fragrance, decorations, etc.). Unit information for Glowbright follows:...
-
EPI is considering outsourcing the production of the handheld control module used with some of its products. The company has received a bid from Control Freak Co. (CFC) to produce 10,000 units of the...
-
Take the diagnostic reasoning situation developed in Table 9.1 and 9.2 of the Dempster Shafer model of Section 9.2.3 and recast it as a Bayesian Belief network. Compare and contrast these two...
-
Convert the following sawhorse formula for one isomer of tartaric acid to a Fischer projection formula. Which isomer of tartaric acid is it? Ho CO2H
-
Complete problem P10-21 using ASPE. Data from P10-21 Original cost ................................................................. $7,000,000 Accumulated depreciation...
-
At what temperature is the rms of Ar equal to that of SF 6 at 298 K? Perform the same calculation for mp .
-
Determine the temperature at which ave for Kr is equal to that of Ne at 298 K.
-
The probability that a particle will have a velocity in the x direction in the range of v x0 and v x0 is given by m? /2kT dv% 1/2 f(-v, s V, s V, ) = 2rkT 1/2 v mv /2kT dv x rkT
-
Identify the at least two main ways to invest in real estate indirectly in your country. *MY COUNTRY IS UNITED STATES * Distinguish between direct and indirect investments in real estate.
-
Question 1 (Marks: 10) According to IAS33 Earnings per share there are two types of shareholder, namely ordinary shareholders and preference shareholders. Q.1.1 Why do we call them preference shares...
-
1. The control principle related to not having the same person approach an invoice and sign the check is known as 2. in terms of internal controls aimed at preventing and detecting fraud, an example...

Study smarter with the SolutionInn App


