Consider the rock salt structure in Figure 13.15. What type of structure would result if all the
Question:
Consider the rock salt structure in Figure 13.15. What type of structure would result if all the anions were somehow removed, leaving only cations?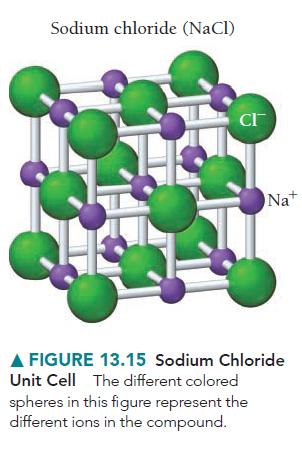
Transcribed Image Text:
Sodium chloride (NaCl) CI™ Na+ A FIGURE 13.15 Sodium Chloride Unit Cell The different colored spheres in this figure represent the different ions in the compound.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
What type of transaction would result in the recording of a prepaid asset? What do you think will happen to that prepaid asset eventually?
-
The marketing manager for Audiotronics Software Company is seeking new market opportunities. He is focusing on the voice recognition market and has narrowed down to three segments: the Fearful...
-
Googles ease of use and superior search results have propelled the search engine to its num- ber one status, ousting the early dominance of competitors such as WebCrawler and Infos- eek. Even later...
-
1. Is religious training mandatory at all of the schools in question? 2. Does the Court see inevitable church-state entanglements if the Board were allowed to exercise jurisdiction over teachers in...
-
In March, William Tackaberry, a real estate agent for Weichert Co. Realtors, informed Thomas Ryan, a local developer, that he knew of property Ryan might be interested in purchasing. Ryan indicated...
-
Analyse the resource-based view of the organisation and describe key concepts related to this approach.
-
Make at least one recommendation on how to build support for your strategy. Tekram is an island nation in the South Pacific. The climate is tropical and the nation is self-governing after a period of...
-
The mean water temperature downstream from a power plant cooling tower discharge pipe should be no more than 100F. Past experience has indicated that the standard deviation of temperature is 2F. The...
-
A company that uses the net method of recording purchases and a perpetual inventory system purchased $2,300 of merchandise on July 5 with terms 2/10, n/30. On July 7, it returned $450 worth of...
-
Danny Imasuen is a 37-year-old student working in Quebec. His wages for the current weekly pay period are $580.00. The employer pays $22.00 for life insurance premiums and $190.00 for group medical...
-
Consider the zinc blende structure in Figure 13.16. What type of structure would result if the remaining tetrahedral sites in the unit cell were also filled with cations? Zinc blende (ZnS) Zn+ S- A...
-
Identify the structure of each of the two unit cells shown in Problem 48 as the rock salt structure, zinc blende structure, fluorite structure, antifluorite structure, or none of these. Problem 48...
-
How does the total contribution margin (unit contribution margin X total number osft a tuenmietnst s s?o ld) differ from the gross margin often seen on companies* financial
-
In your audit of the Whitestable Company's December 31, 1999 financial statements, you become aware of the following controls or procedures over investments, debt, and equity: a. On June 15, 1999,...
-
Christopher Rossi, CPA has been engaged by the Barrington Company, a nonpublicly held manufacturer of children's toys, to review Harrington's December 31, 1999 financial statements. Rossi accepts the...
-
In each of the following cases, (a) identify the aspect of the accounting environment primarily responsible for the ethical pressure on the accountant as pressure to achieve a favorable outcome, to...
-
What is the IRR for an investment of \($1,000\) that yields \($1,300\) in one year?
-
Transit Shuttle Inc. is considering investing in two new vans that are expected to generate combined cash inflows of \($20,000\) per year. The vans combined purchase price is \($65,000\). The...
-
Following are Newman projections for the three tartaric acids (R,R), (S,S), and meso. Which is which? CO,H CO,H OH HO HO CO,H CO H CO H
-
If the cylinder described in Problem 21.3 were initially heated to 500F, how long would it take for the center of the cylinder to cool to 240F if it were constructed of a. Copper? b. Brass? c. Nickel?
-
The speed of sound is given by V sound = ykT / m = yRT / M, where y = Cp / Cv. a. What is the speed of sound in Ne, Kr, and Ar at 1000. K? b. At what temperature will the speed of sound in Kr equal...
-
For O 2 at 1 atm and 298 K, what fraction of molecules has a speed that is greater than v rms ?
-
The escape velocity from the Earths surface is given by vE = (2gR) 1/2 , where g is gravitational acceleration (9.807 m s 2 ) and R is the radius of the Earth (6.37 10 6 m). a. At what temperature...
-
Q 3: (A): How State Bank of Pakistan (SBP) is playing its role in development of Pakistan? What are the major steps taken by SBP in this regard? (B): Due to the economic deterioration in rural areas,...
-
true- false statement (d) Private firms smooth dividends to satisfy shareholders' consumption preferences
-
Abc. Co. has one employee who earns $500 per week and is paid every Monday for the previous week worked. December 31st is a Wednesday. Your employee works Monday-Friday. a. Record the required...

Study smarter with the SolutionInn App


