Identify the structure of each of the two unit cells shown in Problem 48 as the rock
Question:
Identify the structure of each of the two unit cells shown in Problem 48 as the rock salt structure, zinc blende structure, fluorite structure, antifluorite structure, or none of these.
Problem 48
The unit cells for lithium oxide and silver iodide are shown here. Show that the ratio of cations to anions in each unit cell corresponds to the ratio of cations to anions in the formula of each compound.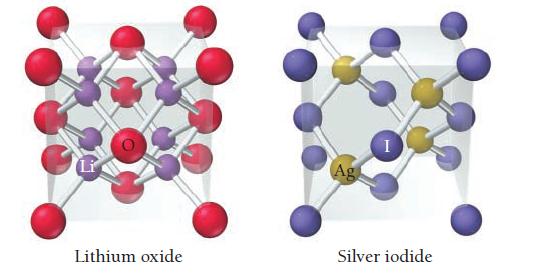
Transcribed Image Text:
Li Lithium oxide Ag Silver iodide
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
Unit cell structure of lithium oxide The unit cell for lithium oxide is the rock salt structure Un...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Identify the structure of each of the two unit cells shown in Problem 47 as the rock salt structure, zinc blende structure, fluorite structure, antifluorite structure, or none of these. Problem 47...
-
The unit cells for lithium oxide and silver iodide are shown here. Show that the ratio of cations to anions in each unit cell corresponds to the ratio of cations to anions in the formula of each...
-
Compound A has molecular formula C10H10O and exhibits a strong signal at 1720 cm1 in its IR spectrum. Treatment with 1,2-ethanedithiol followed by Raney nickel affords the product shown below....
-
discuss case study a bout remote analysis during covid 1 9 - 1 9 virus
-
Insul-Mark is the marketing arm of Kor-It Sales, Inc. Kor-It manufactures roofing fasteners, and Insul-Mark distributes them nationwide. Kor-It contracted with Modern Materials, Inc., to have large...
-
A group of 10 machines is loaded and unloaded by one of three servers. The machines run for an average of six minutes per cycle, and average time to unload and reload is nine minutes. Each time can...
-
State at least one goal and two objectives that are critical to your strategy. Your goal should be SMART (specific, measurable, attainable, realistic, and timely). Tekram is an island nation in the...
-
Before preparing financial statements for the current year, the chief accountant for Toso Company discovered the following errors in the accounts. 1. The declaration and payment of $50,000 cash...
-
I need help with this, Please? 1-WALMART INTERIM AND SEGMENT REPORTING The following information was extracted from quarterly reports for Walmart Inc. (amounts in millions): Three Months Ended April...
-
Draw a flow net for the single row of sheet piles driven into a permeable layer as shown in Figure 8.18. Given: ¢ H1 = 3 m ¢ D = 1.5 m ¢ H2 = 0.5 m ¢ D1 = 3.75 m Calculate the...
-
Consider the rock salt structure in Figure 13.15. What type of structure would result if all the anions were somehow removed, leaving only cations? Sodium chloride (NaCl) CI Na+ A FIGURE 13.15 Sodium...
-
The unit cells for cesium chloride and barium chloride are shown here. Show that the ratio of cations to anions in each unit cell corresponds to the ratio of cations to anions in the formula of each...
-
A cylindrical flask is fitted with an airtight piston that is free to slide up and down, as shown in FIGURE 17-33. A mass rests on top of the piston. The initial temperature of the system is 313 K...
-
Ginger Smalley expects to receive a \($300,000\) cash benefit when she retires five years from today. Ms. Smalleys employer has offered an early retirement incentive by agreeing to pay her...
-
Travis Vintor is seeking part-time employment while he attends school. He is considering purchasing technical equipment that will enable him to start a small training services company that will offer...
-
Medina Manufacturing Company has an opportunity to purchase some technologically advanced equipment that will reduce the companys cash outflow for operating expenses by \($1,280,000\) per year. The...
-
For the following two independent cases, show the cash flows from operating activities section of the 2010 statement of cash flows using the indirect method. Case A Case B 2010 2009 2010 2009 Sales...
-
Construct a network for the project below and find its expected completion time. Activity a b d e f g h i t. (Weeks) 3 5 3 1 3 4 2 3 1 Preceding activities None a a b b, d g, f e, h
-
Which of the following Fischer projection formulas have the same configuration as A, and which are the enantiomer of A? C-H, HO-CH C2H5 b. CHs c. HCH3 . a. H-OH C2Hs
-
An 8.0 kg crate is pulled 5.0 m up a 30 incline by a rope angled 18 above the incline. The tension in the rope is 120 N, and the crates coefficient of kinetic friction on the incline is 0.25. a. How...
-
For N 2 at 298 K, what fraction of molecules has a speed between 200. and 300.m s 1 ? What is this fraction if the gas temperature is 500. K?
-
A molecular beam apparatus employs supersonic jets that allow gas molecules to expand from a gas reservoir held at a specific temperature and pressure into a vacuum through a small orifice. Expansion...
-
Demonstrate that the MaxwellBoltzmann speed distribution is normalized.
-
Your company has purchased equipment worth $40,000 and would like to compare the impact of straight-line depreciation versus accelerated depreciation (double declining method). The equipment has a...
-
You have $22,000 to invest. You want to purchase shares of Alaska Air at $43.76, Best Buy at $52.62, and Ford Motor at $9.16. How many shares of each company should you purchase so that your...
-
Q 1: (A) What are open market operations? How do these work as a method of credit control? (B): What is the likely impact of money creation by the commercial banks on national income? explain briefly...

Study smarter with the SolutionInn App


