The unit cells for lithium oxide and silver iodide are shown here. Show that the ratio of
Question:
The unit cells for lithium oxide and silver iodide are shown here. Show that the ratio of cations to anions in each unit cell corresponds to the ratio of cations to anions in the formula of each compound.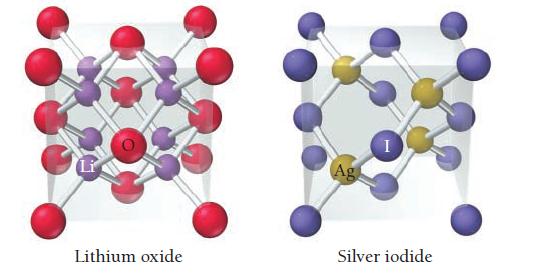
Transcribed Image Text:
Li Lithium oxide Ag Silver iodide
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
Lithium oxide Li2O The unit cell for lithium oxide contains four lithium cations Li and four oxide a...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Identify the structure of each of the two unit cells shown in Problem 48 as the rock salt structure, zinc blende structure, fluorite structure, antifluorite structure, or none of these. Problem 48...
-
Identify the structure of each of the two unit cells shown in Problem 47 as the rock salt structure, zinc blende structure, fluorite structure, antifluorite structure, or none of these. Problem 47...
-
The unit cells for cesium chloride and barium chloride are shown here. Show that the ratio of cations to anions in each unit cell corresponds to the ratio of cations to anions in the formula of each...
-
Explain why placing soy sauce in airtight bottles was more successful for long-distance shipping than simply placing the sauce in barrels.
-
On November 1, the Kansas City Post Office Employees Credit Union merged into the Kansas City Telephone Employees Credit Union to form the Communications Credit Union (Credit Union). Systems Design...
-
Given the data in problem 3, is a moving average a suitable method of forecasting? Compute the Mean Absolute Percentage Error, the Mean Absolute Deviation, and the Mean Square Deviation. Problem 3...
-
An annual report of The Campbell Soup Company reported on its income statement $2.4 million as equity in earnings of affiliates. Journalize the entry that Campbell would have made to record this...
-
The earnings, dividends, and stock price of the Abbott Company are expected to grow at 9 percent per year. Abbott's common stock sells for $30 per share, and the company will pay a year-end dividend...
-
A Moving to another question will save this response. Question 9 Why would a derivative investor write a "put option To earn income Limit losses from a decline in the underlying shares Lock in the...
-
The government has just increased taxes. a. Demonstrate the effect on the price level and output in the standard model. b. How would your answer to a differ if there were partial crowding out? c. How...
-
Consider the rock salt structure in Figure 13.15. What type of structure would result if all the anions were somehow removed, leaving only cations? Sodium chloride (NaCl) CI Na+ A FIGURE 13.15 Sodium...
-
An oxide of rhenium crystallizes with the unit cell shown here (rhenium = gray; oxygen = red). What is the formula of the oxide?
-
Jones Legal Investigation Services, Inc., is a growing business that performs legal investigations at the request of attorneys. The company owner, Richard Jones, offers a wide range of investigative...
-
Assume that two investments are combined in a portfolio. a. In words, what is the expected rate of return on the portfolio? b. What condition must be present for the portfolio to have lower risk than...
-
Several years ago, the Value Line Investment Survey reported the following market betas for the stocks of selected healthcare providers: At the time these betas were developed, reasonable estimates...
-
Suppose that the risk-free rate, RF, is 8 percent and the required rate of return on the market, R(R M ), is 14 percent. a. Write out the security market line (SML) equation, and explain each term....
-
Verify that under the mutual independent log-linear model (7.23), the variable \(y\) is independent with the other two. log ijk = log +logi+k+log +j+ = X + X + x + X + X (7.23)
-
Ted's Bike Shop sells new and used bicycle parts. Although a majority of its sales are cash sales, it makes a significant amount of credit sales. During 2011, its first year of operations, Ted's Bike...
-
4-Chloro-2-pentene has a double bond that can have either the E or the Z configuration and a stereogenic center that can have either the R or the S configuration. How many stereoisomers are possible...
-
Apply Jacobis method to the given system. Take the zero vector as the initial approximation and work with four-significant-digit accuracy until two successive iterates agree within 0.001 in each...
-
Using the distribution of particle translational kinetic energy provided in Problem P33.19, derive an expression for the fraction of molecules that have energy greater than some energy . The rate...
-
Imagine a cubic container with sides 1 cm in length that contains 1 atm of Ar at 298 K. How many gaswall collisions are there per second?
-
The vapor pressure of various substances can be determined using effusion. In this process, the material of interest is placed in an oven (referred to as a Knudsen cell) and the mass of material lost...
-
Holding other variables constant, a recession in the US economy should: Select one: A) cause a depreciation of the dollar because the nominal interest rate in the United States would increase b)...
-
CROSS RATES The table below gives the exchange rates between U.S. dollar, British pound and Swedish krona. What is the exchange rate between Swedish kronas and pounds? Round your answer to two...
-
Presented below is pension information related to Blossom Company as of December 31, 2021: Accumulated benefit obligation Projected benefit obligation Plan assets (at fair value) Accumulated OCI...

Study smarter with the SolutionInn App


