Consider this set of ionization energies. To which third-period element do these ionization values belong? IE =
Question:
Consider this set of ionization energies.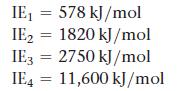
To which third-period element do these ionization values belong?
Transcribed Image Text:
IE₁ = 578 kJ/mol IE2 = 1820 kJ/mol IE3 = 2750 kJ/mol IE4 11,600 kJ/mol =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
The ionization energies provided seem to represent successive ionization energies and the large incr...View the full answer

Answered By

Wahome Michael
I am a CPA finalist and a graduate in Bachelor of commerce. I am a full time writer with 4 years experience in academic writing (essays, Thesis, dissertation and research). I am also a full time writer which assures you of my quality, deep knowledge of your task requirement and timeliness. Assign me your task and you shall have the best.
Thanks in advance
4.90+
63+ Reviews
132+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The Crazy Eddie fraud may appear smaller and gentler than the massive billion-dollar frauds exposed in recent times, such as Bernie Madoffs Ponzi scheme, frauds in the subprime mortgage market, the...
-
Read the case study "Southwest Airlines," found in Part 2 of your textbook. Review the "Guide to Case Analysis" found on pp. CA1 - CA11 of your textbook. (This guide follows the last case in the...
-
THIRD AVENUE SOFTWARE HEALTH-CARE APP PROJECT This case is new for the ninth edition of Information Technology Project Management . The case provides an opportunity to apply agile and Scrum...
-
The Litzenberger Company has projected the following quarterly sales amounts for the coming year: a. Accounts receivable at the beginning of the year are $310. Litzenberger has a 45-day collection...
-
The stockholders equity section of Platt Corporations balance sheet consists of common stock ($10 par) $1,000,000 and retained earnings $300,000. A 10% stock dividend (10,000 shares) is declared when...
-
Air enters a rectangular duct at T1 = 300 K, P1 = 420 kPa, and Ma1 = 2. Heat is transferred to the air in the amount of 55 kJ/kg as it flows through the duct. Disregarding frictional losses,...
-
18.3 In September 2009 Kevin sells a drawing for 2,000. He bought the drawing in February 2003 for 50,000 when it was thought (incorrectly) to be by a famous artist. Compute the allowable loss.
-
Samantha Stamford opened a medical practice. During July, the first month of operation, the business, titled Samantha Stamford, M.D., experienced the following events: July 6 Stamford contributed...
-
Jay Banning, CEO and a major stockholder of Banning Incorporated, was unhappy with its operating results for the past year. The company manufactures two environmentally friendly industrial caliber...
-
Honda Motor Corporation of Japan is a leading international manufacturer of automobiles, motorcycles, all-terrain vehicles, and personal watercraft. As a Japanese company, it follows Japanese GAAP...
-
For each element, predict where the jump occurs for successive ionization energies. (For example, does the jump occur between the first and second ionization energies, the second and third, or the...
-
Arrange these elements in order of decreasing first ionization energy: Cl, S, Sn, Pb.
-
Analytical procedures are generally used to produce evidence from a. Confirmations mailed directly to the auditors by client customers. b. Physical observation of inventories. c. Relationships among...
-
Nervousness and depression are examples of __________ symptoms. psychophysiologic social health environmental psychological Roberta is using the structured format to present the results of his study...
-
Identify the stage of change the client is in and write in behavioral language at least one problem, with at least one goal and a minimum of two objectives for each goal for the client vignettes...
-
Photon Technologies, Inc., a manufacturer of batteries for mobile phones, signed a contract with a large electronics manufacturer to produce three models of lithium-ion battery packs for a new line...
-
C 2 H 6 O 2 + NaOH + 6 H 2 O C 2 H 3 NaO 3 + O 2 + 3 H 2Hydrogen is produced at the cathode, oxYGEN AT THE ANODE .Mass balance to produce 5000 tonnes a year of glycolic acid, formic acid and oxalic...
-
Please answer: a discussion of the ethical issues involved. The court might not itself consider the ethics of the actions of the parties. However, I ask that you consider the ethics of the following:...
-
What is the present value concept and why is it important in capital budgeting?
-
Which of the following is FALSE regarding the purchasing power parity (PPP). a. The PPP is a manifestation of the law of one price b. The PPP says that a country with a higher expected inflation can...
-
Is A a linear operator if A f (x) = d 2 f (x) / dx 2 + xf (x)?
-
Two operators can be applied to a function in succession. By definition, AB f(x) = A [B f(x)]. Evaluate AB f(x) if A = d /dx, B = x, and f (x) = cos x.
-
Cos x an eigenfunction of the operator A if A f (x) = xf (x)?
-
Perpetual inventory using FIFO The following units of a particular item were available for sale during the calendar year: Jan. 1 Inventory 3,700 units at $39 Apr. 19 Sale 2,400 units June 30 Purchase...
-
7. In preparing the monthly bank reconciliation, Fur Ltd ascertains that there is a $750 cheque from Hunt Traders for merchandise that is marked NSF. The journal entry to record this in Fur Ltd's...
-
1. Peter, age 25, is single. He lives with his disabled and dependent mother, for whom he provides more than a half of support. Peter earned a $75,000 salary. His other income consisted of $2,000...

Study smarter with the SolutionInn App


