Determine the composition, relative amounts, and phases present at point B on Figure 25.7. Temperature (C) 1900
Question:
Determine the composition, relative amounts, and phases present at point B on Figure 25.7.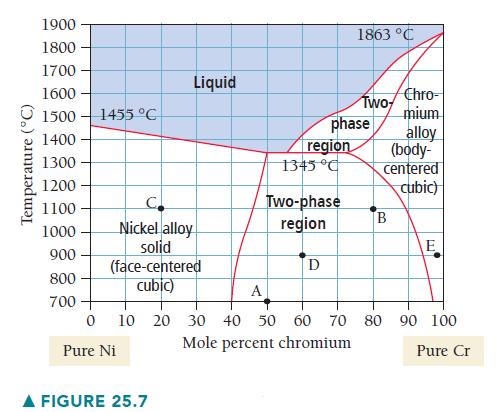
Transcribed Image Text:
Temperature (°C) 1900 1800 1700 1600- 1500 1455 °C 1400 1300 1200 1100 1000 900 800 700 C Nickel alloy solid (face-centered cubic) 0 Pure Ni Liquid A FIGURE 25.7 region 1345 °C phase Two-phase region D 1863 °C Two Chro- mium B alloy (body- centered cubic) E A 10 20 30 40 50 60 70 80 90 100 Mole percent chromium Pure Cr
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
Point B represents 80 mol Cr at 1100 C Point B is lo...View the full answer

Answered By

Vijesh J
My passion to become a tutor is a lifetime milestone. Being a finance and marketing professional with hands-on experience in wealth management, portfolio management, team handling and actively contributing in promoting the company. Highly talented in managing and educating students in most attractive ways were students get involved. I will always give perfection to my works. Time is the most important for the works and I provide every answer on time without a delay. I will proofread each and every work and will deliver a with more perfection.
4.70+
5+ Reviews
15+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
An alloy is a metallic mixture composed of two or more elements. As is the case in all mixtures, the relative amounts of the elements in an alloy can vary. In some cases, the components of an alloy...
-
Determine the composition, relative amounts, and phases present at point C on Figure 25.7. Temperature (C) 1900 1800 1700 1600- 1500 1455 C 1400 1300 1200 1100 1000 900 800 700 C Nickel alloy solid...
-
Determine the composition and phase present at point B on Figure 25.5. Temperature 1500 1400 1300 1200- 1100 1000- 900 Liquid 1084 C A D Solid alloy C B. 1455 C 0 10 20 30 40 50 60 70 80 90 100 Mole...
-
An air-filled X-band rectangular waveguide has dimensions a = 2.286 cm and b = 1.016 cm. If the waveguide has copper walls ( = o , = o , = 5.8 x 10 7 S/m), find the attenuation in dB/m due to the...
-
The stockholders equity section of the balance sheet of Rago Corporation as of December 31, 2010, was as follows: Contributed capital Common stock , $4 par value, 250,000 shares authorized, 100,000...
-
Traveling across the country, Paul and his friend Randy often stopped at restaurants with familiar brands. One advantage of this is that the menu and often the service quality are likely to be...
-
Who burns more energy? Table 14.1 gives data on the lean body mass and metabolic rate for 12 women and 7 men. You made a scatterplot of these data in Exercise 14.14. (a) Do you think the correlation...
-
Taser International, Inc., manufactures a product commonly known as a taser. Tasers have prongs or cords that emit high voltage electrical currents that, when they touch a persons body, immobilize...
-
Novak Corporation reported net income of $338,000 in 2020 and had 54,200 shares of common stock outstanding throughout the year. Also outstanding all year were 5,400 shares of cumulative preferred...
-
List three categories of metallurgical processes.
-
Which type of metallurgical process uses electrolysis to extract a metal from its ore? (a) Pyrometallurgy (b) Hydrometallurgy (c) Powder metallurgy (d) Electrometallurgy
-
Use a graph to find a number such that |4x + 1 3| < 0.5 whenever |x 2|
-
Reflect on your semester. How do you plan onmeasuringyour professionalgrowth in the future? What were the most challenging topics to you? What topics felt more intuitive/easy? How do you plan on...
-
Aside from shareholders, who do you believe is the second stakeholder in whose interests the company should be concerned? Justify your response What will you do to ensure the company's success...
-
a) What CSR did your organization do - how did it improve your organization's image? b) If your organization did not do any CSR, as the boss, what CSR activities would you suggest doing and why?
-
Do you believe NIL promotes "love of the game," or does it make college sports more about money and business? What are the most significant positive and negative effects of NIL, in your opinion? What...
-
Even well-managed organizations do not always work as efficiently and effectively as management would like. At Hewlett-Packard (HP), billions of dollars of product are being shipped - from computers...
-
Compute trend percentages for valley View Sales & Service's total revenue and net inceom for the following fve-tear period, using year 0 as the base year. Eound to the narest full percent. Which grew...
-
A bubble-point liquid feed is to be distilled as shown in Figure. Use the Edmister group method to estimate the mole-fraction compositions of the distillate and bottoms. Assume initial overhead and...
-
Figure Q20.7 shows the direction of B(vector) in a particular region of space. The density (i.e., spacing) of the crosses and dots indicate qualitatively the magnitude of B(vector). What is the...
-
In our discussion of the solenoid in Figure 20.33B, we claimed that the field outside the solenoid is much smaller than the field inside and that it is a good approximation to assume the field...
-
Two parallel wires are oriented perpendicular to the page as shown in Figure Q20.9. The wires carry equal currents, and the direction of the current is into the page for each. The vertices of an...
-
When credit terms for a sale are 2/15, n/40, the customer saves by paying early. What percent (rounded) would this savings amount to on an annual basis
-
An industrial robot that is depreciated by the MACRS method has B = $60,000 and a 5-year depreciable life. If the depreciation charge in year 3 is $8,640, the salvage value that was used in the...
-
What determines a firm's beta? Should firm management make changes to its beta? Be sure to consider the implications for the firm's investors using CAPM.

Study smarter with the SolutionInn App


