Determine the composition, relative amounts, and phases present at point C on Figure 25.7. Temperature (C) 1900
Question:
Determine the composition, relative amounts, and phases present at point C on Figure 25.7.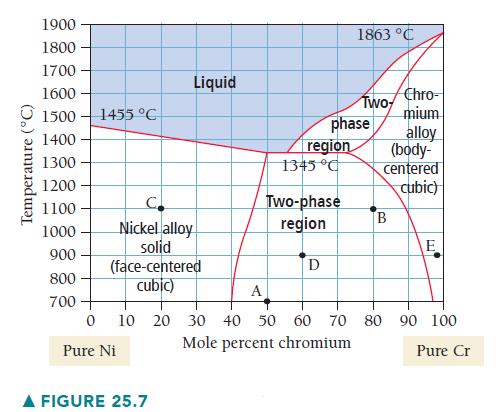
Transcribed Image Text:
Temperature (°C) 1900 1800 1700 1600- 1500 1455 °C 1400 1300 1200 1100 1000 900 800 700 C Nickel alloy solid (face-centered cubic) 0 Pure Ni Liquid A FIGURE 25.7 region 1345 °C phase Two-phase region D 1863 °C Two Chro- mium B alloy (body- centered cubic) E A 10 20 30 40 50 60 70 80 90 100 Mole percent chromium Pure Cr
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
Point C represents 20 mol Cr at 1100 C Point C is located ...View the full answer

Answered By

Muhammad Haroon
More than 3 years experience in teaching undergraduate and graduate level courses which includes Object Oriented Programming, Data Structures, Algorithms, Database Systems, Theory of Automata, Theory of Computation, Database Administration, Web Technologies etc.
5.00+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Determine the composition, relative amounts, and phases present at point B on Figure 25.7. Temperature (C) 1900 1800 1700 1600- 1500 1455 C 1400 1300 1200 1100 1000 900 800 700 C Nickel alloy solid...
-
1. How strong are the competitive forces confronting J. Crew in the market for specialty retail? Do a [Michael Porter] five-forces analysis to support your answer. (see chapter 3 in the textfor...
-
Determine the composition, relative amounts, and phases present at points C and D on the CoCu phase diagram (see Problem 37 for phase diagram). Problem 37 Determine the composition, relative amounts,...
-
The following are some transactions of Surendal Company for 2024. Surendal Company uses straight-line depreciation and has a December 31 year end. Apr. 1 Retired a piece of equipment that was...
-
On Tuesday, January 19, 1988, IBM reported greatly increased earnings for the fourth quarter of 1987. Despite this reported gain in earnings, the price of IBMs stock on the New York Stock Exchange...
-
What determines whether or not to issue an unqualified audit opinion on the compliance of a set of financial statements with IFRS?
-
Who burns more energy? Metabolic rate, the rate at which the body consumes energy, is important in studies of weight gain, dieting, and exercise. Table 14.1 gives data on the lean body mass and...
-
In the past, the rules of discovery were very restrictive, and trials often turned on elements of surprise. For example, a plaintiff would not necessarily know until the trial what the defendants...
-
The Reef Corporation has an opportunity to sell shark repellant. Oscar, the CEO, would like Sykes, the vice president, to evaluate the project. Oscar owns all 5 0 , 0 0 0 outstanding shares of the...
-
Why is Ni not considered a common metal, even though it composes over 2% of the total mass of Earth and only Fe and Mg have a higher percent composition of Earths total mass?
-
An interstitial alloy contains a nonmetal (X) that occupies one-eighth of the tetrahedral holes of the cubic closest-packed structure of the metal (M). What is its formula? (a) MX (b) M 2 X (c) MX 2...
-
How far apart are two charges of +1 10 8 C that repel each other with a force of 0.1 N?
-
On Apple company with specific iPhone product Required to conduct a SWOT and PESTEL analysis, identifying the internal strengths and weaknesses and external opportunities and threats of the Apple...
-
In which social platforms are Walmart's brand/company active? In your opinion, are they doing a good job regarding customer engagement through social media channels? (Required: screenshots from the...
-
After you have watched both films, how would you describe each film? Also, consider what makes these early films different. List as many observations as you can that separate the Lumi re brothers...
-
How to develop the following points with the Poshmark application for second hand? 1. What are the main reasons for using this product? Or why not? 2. What are the hidden motivations? 3. Are there...
-
Suppose, in an experiment to determine the amount of sodium hypochlorite in bleach, you titrated a 22.84 mL sample of 0.0100 M K I O 3 with a solution of N a 2 S 2 O 3 of unknown concentration. The...
-
Identify any weak¬nesses revealed by the statement of cash flows of Sunshine Fruit, Inc. Sunshine Fruit, Inc. Statement of Cash Flows For the Current Year Operating activities Net income 104,000...
-
Consider the setup in Problem 16. Show that the relative speed of the ball and the point of contact on the stick is the same before and immediately after the collision. (This result is analogous to...
-
Consider the configuration of resistors shown in Figure P19.108. Each connection can have a resistor that is disconnected or whose value is 5.0 or 10.0. To determine the value of each resistor or...
-
Consider again the configuration of resistors shown in Figure P19.108. As in Problem 108, each connection can have a resistor that is disconnected or whose value is 5.0 or 10.0 . This time, you...
-
Electrical wire comes in different sizes (different diameters), which are referred to according to their gauge number. For example, a 16-gauge wire has a diameter of approximately 1.29 mm and is...
-
Phantom Consulting Inc. is a small computer consulting business. The company is organized as a corporation and provides consulting services, computer system installations, and custom program...
-
Sam owns a 25% in Spade, LLC. In 2021, Spade reports $100,000 or ordinary income. What is Sams qualified business income (QBI) deduction? answer is 5,000 but please show how to get it
-
crane Inc. common chairs currently sell for $30 each. The firms management believes that it's share should really sell for $54 each. If the firm just paid an annual dividend of two dollars per share...

Study smarter with the SolutionInn App


