Determine the formal charge of nitrogen in the structure shown here: a) +1 H: H-C-N-8: b) +2
Question:
Determine the formal charge of nitrogen in the structure shown here: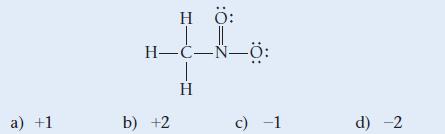
Transcribed Image Text:
a) +1 HÖ: H-C-N-8: b) +2 H c) -1 d) -2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)

Answered By

Murshidha parveen
I have a degree in Business Administration from the National University of Córdoba. I've been working as an online tutor for the past four years. I specialize in history and business articles. Every day I help students to do their homework, complete projects, dispel doubts, and much more. I hope to be a force in helping you get the knowledge you need.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
In N 2 O, nitrogen is the central atom, and the oxygen atom is terminal. In OF 2 , however, oxygen is the central atom. Use formal charges to explain why. Formal charge = number of valence electrons...
-
Consider the Lewis structure for the polyatomic oxyanion shown here, where X is an element from the third period (Na --- Ar). By changing the overall charge, n, from 1 -- to 2 --- to 3 --- we get...
-
Identify the tort or possible tort claim. Defamation Libel Describe the essential elements of the tort or possible tort claim. These elements are provided by law. It is not necessary in response to...
-
In its first year of operation, Oriole Printing Shop estimated manufacturing overhead costs and activity in order to determine a predetermined overhead rate. At year end, March 31, overhead was...
-
Sonne Company produces a perfume called Whim. The direct materials and direct labor standards for one bottle of Whim are given below: During the most recent month, the following activity was...
-
List the main elements of balance sheet and income statement.
-
Describe the stages in which conflict develops.
-
Nellie works for a large Fortune 500 company. She heads the information systems department and works closely with the accounting department. The company works with many associates. They have many...
-
Cricket Green invests $1,500 a year at the beginning of each year for a total of 20 years at 7.5 percent annual rate. How much money will Cricket have 20 years from now? $69,829 $71,329 $60,529...
-
Refer to Exercise 2.20. Another pair of students achieved the following marks. Student P: 94, 91, 88, 77, 75, 74, 66, 65, 54, 52 Student Q: 93, 91, 88, 75, 75, 73, 67, 66, 55, 51 a. Calculate the...
-
Write the Lewis structure for XeF 2 .
-
Why is the formation of solid sodium chloride from solid sodium and gaseous chlorine exothermic, even though it takes more energy to form the Na + ion than the amount of energy released upon...
-
Explain which compound has a faster rate of SN1 reaction. a) c) HC CI CI or or J d) CI CHCI or or D CHCl OCH 3
-
Description: duff owes relatives $13,000 for college loans. find the required quarterly payment into a sinking fund if duff pays off the loan in 3 years and the interest rate is 8% per year...
-
1 3 , 9 5 0 ) Repairs and Maintenance ( $ 2 , 8 5 0 ) Utilities Expense ( $ 8 8 0 ) Operating Income $ 1 0 , 2 4 2 Other Income - Gain on Sale $ 3 0 0 Interest Expense ( $ 2 5 0 ) Earnings Before...
-
Description: The company currently has outstanding a bond with a 5.5 percent coupon rate and another bond with a 3.5 percent coupon rate. The firm has been informed by its investment banker that...
-
Find the equation of line joining the points (4, -3) and (-2, 7).
-
Calculate the work of reversible expansion of 1 mole of ideal gas at 25 degree celsius from 10 L to 20 L.
-
In the table, weight gain-time data for the oxidation of copper at an elevated temperature are tabulated. W (mg/cm2) Time (min) 0.316........................... 15 0.524........................... 50...
-
Based on the scenario described below, generate all possible association rules with values for confidence, support (for dependent), and lift. Submit your solutions in a Word document (name it...
-
An A-36-steel hoop has an inner diameter of 23.99 in., thickness of 0.25 in., and width of 1 in. If it and the 24-in.-diameter rigid cylinder have a temperature of 65° F, determine the...
-
A pressure-vessel head is fabricated by welding the circular plate to the end of the vessel as shown. If the vessel sustains an internal pressure of 450 kPa, determine the average shear stress in the...
-
The gas pipe line is supported every 20 ft by concrete piers and also lays on the ground. If there are rigid retainers at the piers that hold the pipe fixed, determine the longitudinal and hoop...
-
Suppose you sell a fixed asset for $82,950 when its book value is $42,542. If your company's marginal tax rate is 25 percent, what will be the after-tax cash flow of this sale?
-
Imagine you run an airline company. An advisor tells you that Hedging against increases in jet fuel prices can sometimes erode the competitive position of an airline. Provide two examples where you...
-
Delaying the payment of accrued expenses until a later period is a technique that management can use to manipulate the current years cash flow from operating activities. True or False

Study smarter with the SolutionInn App


