Determine whether each reaction is a redox reaction. For each redox reaction, identify the oxidizing agent and
Question:
Determine whether each reaction is a redox reaction. For each redox reaction, identify the oxidizing agent and the reducing agent.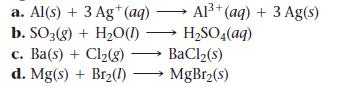
Transcribed Image Text:
a. Al(s) + 3 Ag+ (aq) b. SO3(g) + H₂O(1)→ c. Ba(s) + Cl₂(g) d. Mg(s) + Br₂(1) Al³+ (aq) + 3 Ag(s) H₂SO4(aq) → BaCl₂(s) MgBr₂(s)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
a Als 3 Ag aq Al3aq 3 Ags This is a redox reaction The aluminum metal ...View the full answer

Answered By

Nazrin Ziad
I am a post graduate in Zoology with specialization in Entomology.I also have a Bachelor degree in Education.I posess more than 10 years of teaching as well as tutoring experience.I have done a project on histopathological analysis on alcohol treated liver of Albino Mice.
I can deal with every field under Biology from basic to advanced level.I can also guide you for your project works related to biological subjects other than tutoring.You can also seek my help for cracking competitive exams with biology as one of the subjects.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Determine whether each reaction is a redox reaction. For each redox reaction, identify the oxidizing agent and the reducing agent. a. 4 Li(s) + O(g) 2 LiO(s) b. Mg(s) + Fe+ (aq) Mg2+(aq) + Fe(s)...
-
Consider the redox reaction (a) Identify the oxidizing agent on the left side of the reaction and write a balanced oxidation half-reaction. (b) Identify the reducing agent of the left side of the...
-
stock options are no longer as valuable and employees in startups are losing out to founders and early investors for all of these reasons except: there is higher employee turnover, less of a delay in...
-
j) Assume that one of these portfolio's is the Market Portfolio and all portfolios, except Portfolio G, are fairly priced according to the CAPM. Derive the Treynor Measure for these fairly priced...
-
The Gayton Menswear company was founded by Fred Williams in 1986 and has grown steadily over the years. Fred now has 17 stores located throughout the central and northern parts of the state. Because...
-
In 2019, the Indonesian government threatened to pull out of the Paris Climate Agreement and thereby bring a World Trade Organization challenge in their ongoing tariff trade spat with the European...
-
What procedures should be used to develop the new processes for using the company s intranet for communication with customers?
-
Laredo Leather Company manufactures high-quality leather goods. The companys profits have declined during the past nine months. In an attempt to isolate the causes of poor profit performance,...
-
KUALA LUMPUR: Malaysian palm oil futures fell 0.42% on Wednesday, as concerns over rising new Covid-19 coronavirus infections weighed on investors mood. The benchmark palm oil contract for September...
-
Wayland Custom Woodworking is a firm that manufactures custom cabinets and woodwork for business and residential customers. Students will have the opportunity to establish payroll records and to...
-
Determine whether each redox reaction occurs spontaneously in the forward direction. a. Ni(s) + Zn+ (aq) b. Ni(s) + Pb+ (aq) c. Al(s) + 3 Ag+ (aq) d. Pb(s) + Mn+ (aq) 2+ Ni+(aq) + Zn(s) Ni+(aq) +...
-
What is the oxidation state of Cl in each ion? a. CIO- b. ClO c. ClO3 d. CIO4
-
Gold Corporation, a calendar year C corporation, was formed in 2011 and has been profitable until the current year. In 2017, Gold incurs a net operating loss. Identify the issues that Gold...
-
Discuss the Competitive Markets and Externalities simulations (both with and without policy interventions) . What impact do policy interventions have on the supply and demand equilibrium for a...
-
The best consultant to fix issue number one is Frederick Taylor who is credited with creating the scientific management movement (Lumen, n.d.). Since Taylor's work focused on how a process could be...
-
1. Which Pepsico products are growing faster than soft drinks (why) and by what percentage? 2. Why do the fastest growing products experience a more complex supply chain? Explain. 3. What are some of...
-
Use BLUF (Bottom Line UP Front) or Brief for answering the following questions: 1) There are a number of InfoSec frameworks / models available in industry. A. What is an InfoSec framework / model? B....
-
An introduction to organizational structure. Topics such as alternative organizational structures, the reciprocal relationship between multinational strategy and structure, and how recourses affect...
-
At 500C (930F), what is the maximum solubility (a) of Cu in Ag? (b) Of Ag in Cu?
-
Element compound homogeneous mixture (heterogeneous mixture) 4) A piece of gold has a mass of 49.75 g. What should the volume be if it is pure gold? Gold has a density of 19.3 g/cm (3 points) D=m/v...
-
The viscosity of an oil is given as 80 SUS at 100F. Determine the viscosity in m 2 /s.
-
Convert a viscosity measurement of 6.5 10 -3 Pas into the units of lbs/ft 2 .
-
An oil container indicates that it has a viscosity of 0.12 poise at 60C. Which oil in Appendix D has a similar viscosity?
-
Maria is considering purchasing the stock of Ceci Manufacturing. What should Maria be willing to pay for Ceci today if it is expected to pay a $3.97 dividend in one year and she expects dividends to...
-
LUCENT TECHNOLOGIES AT&T spun off its research and development division (the former Bell Laboratories) in April of 1996, and the newly independent company - renamed Lucent Technologies - was an...
-
An equally-weighted portfolio contains eight securities, each with a standard deviation of returns of 50%. If the pairwise correlation of returns for these securities is 0.6, calculate the resulting...

Study smarter with the SolutionInn App


