Determine whether each reaction is a redox reaction. For each redox reaction, identify the oxidizing agent and
Question:
Determine whether each reaction is a redox reaction. For each redox reaction, identify the oxidizing agent and the reducing agent.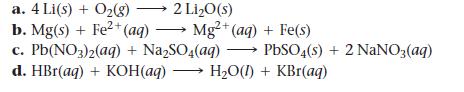
Transcribed Image Text:
a. 4 Li(s) + O₂(g) → 2 Li₂O(s) b. Mg(s) + Fe²+ (aq)→ Mg2+(aq) + Fe(s) c.Pb(NO3)2(aq) + Na₂SO4(aq) d. HBr(aq) + KOH(aq)→ H₂O(1) + KBr(aq) PbSO4(s) + 2NaNO3(aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (2 reviews)
a Redox reaction oxidizing agen...View the full answer

Answered By

Ajeet Singh
Hi there! Are you looking for a committed, reliable, and enthusiastic tutor? Well, teaching and learning are more of a second nature to me, having been raised by parents who are both teachers. I have done plenty of studying and lots of learning on many exciting and challenging topics. All these experiences have influenced my decision to take on the teaching role in various capacities. As a tutor, I am looking forward to getting to understand your needs and helping you achieve your academic goals. I'm highly flexible and contactable. I am available to work on short notice since I only prefer to work with very small and select groups of students. Areas of interest: Business, accounting, Project management, sociology, technology, computers, English, linguistics, media, philosophy, political science, statistics, data science, Excel, psychology, art, history, health education, gender studies, cultural studies, ethics, religion. I am also decent with math(s) & Programming. If you have a project you think I can take on, please feel welcome to invite me, and I'm going to check it out!
5.00+
4+ Reviews
24+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Determine whether each reaction is a redox reaction. For each redox reaction, identify the oxidizing agent and the reducing agent. a. Al(s) + 3 Ag+ (aq) b. SO3(g) + HO(1) c. Ba(s) + Cl(g) d. Mg(s) +...
-
Consider the redox reaction (a) Identify the oxidizing agent on the left side of the reaction and write a balanced oxidation half-reaction. (b) Identify the reducing agent of the left side of the...
-
stock options are no longer as valuable and employees in startups are losing out to founders and early investors for all of these reasons except: there is higher employee turnover, less of a delay in...
-
1. Consider a household that has $300 to spend on back-to- school shoes. They visit one of the discount shoe stores where shoes are buy one pair for $50, get the second half off. a. Draw the budget...
-
The Palmer Company manufactures various types of clothing products for women. To accumulate the costs of manufacturing these products, the companys accountants have established a computerized cost...
-
A trial balance was extracted on 31 December 2008 and the totals did not agree, there being a 90 shortage on the debit column. As a result, a suspense account was opened. In January 2009, the...
-
What process should Stacey and her team use to determine which lunch - building tactics should be implemented?
-
Zagats publishes restaurant ratings for various locations in the United States. The file Restaurants contains the Zagat rating for food, dcor, service, and the cost per person for a sample of 100...
-
Gull Corporation, a cash method, calendar year C corporation, was formed and began business on November 1 , 2 0 2 3 . Gull incurred the following expenses during its first year of operations (...
-
In 2017, Caitlyn Smith's husband Ben passed away. The next year she moved from Louisiana to Texas. Caitlyn has not remarried as of the end of 2019 and currently lives in Portland, TX with her three...
-
Determine whether each redox reaction occurs spontaneously in the forward direction. a. Ni(s) + Zn+ (aq) b. Ni(s) + Pb+ (aq) c. Al(s) + 3 Ag+ (aq) d. Pb(s) + Mn+ (aq) 2+ Ni+(aq) + Zn(s) Ni+(aq) +...
-
What is the oxidation state of Cl in each ion? a. CIO- b. ClO c. ClO3 d. CIO4
-
Do you think firms in certain cultures prefer to conduct certain types of market research? Explain.LO12
-
The four classic leadership styles There are four leadership styles which are prominent in today's businesses and companies. They are Laissez-faire, Autocratic, Democratic, and Charismatic...
-
How can conflict be viewed positively? Cite a specific example of when this might be the case. Under what circumstances might "avoiding" conflict be "managing" conflict? In other words, when might...
-
Visit the website and answer the questions below. https://www.forbes.com/advisor/business/software/best-crm-small-business/ Based on the CRM software discussed in the article, which 3 software...
-
Planning consists of translating and organizations mission and vision into objectives. The organization's purpose is expressed as a mission statement, and what it becomes is expressed as a vision...
-
Question 1- Visit the Boots and Hearts Festival website: www.bootsandhearts.com. Using the information you find on the site, make an analysis of the festival's Strengths, Weaknesses, Opportunities...
-
For alloys of two hypothetical metals A and B, there exist an α, A-rich phase and a β, B-rich phase. From the mass fractions of both phases for two different alloys provided...
-
One study found that the elderly who do not have children dissave at about the same rate as the elderly who do have children. What might this finding imply about the reason the elderly do not dissave...
-
In a falling-ball viscometer, a steel ball 1.6 mm in diameter is allowed to fall freely in a heavy fuel oil having a specific gravity of 0.94. Steel weighs 77 kN/m 3 . If the ball is observed to fall...
-
A capillary tube viscometer similar to that shown in Fig. 2.7 is being used to measure the viscosity of an oil having a specific gravity of 0.90. The following data apply: Tube inside diameter = 2.5...
-
In a falling-ball viscometer, a steel ball with a diameter of 0.063 in is allowed to fall freely in a heavy fuel oil having a specific gravity of 0.94. Steel weighs 0.283 lb/in 3 . If the ball is...
-
Which one of the following is correct about Modigliani and Miller's Proposition I A. the market value of any firm is independent of its capital structure B. the market value of a firm's debt is...
-
(Future value)If you deposit $2,300 today into an account earning an annual rate of return of 11 percent, what would your account be worth in 20 years (assuming no further deposits)? In 25 years?...
-
You receive a cash dividend of $700 that is fully franked (i.e. has the maximum amount of franking credits attached). In relation to this dividend, the total amount that you must include in your...

Study smarter with the SolutionInn App


