Determine whether each redox reaction occurs spontaneously in the forward direction. a. Ca+ (aq) + Zn(s) b.
Question:
Determine whether each redox reaction occurs spontaneously in the forward direction.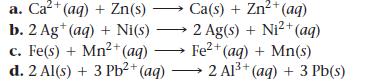
Transcribed Image Text:
a. Ca²+ (aq) + Zn(s) b. 2 Ag+ (aq) + Ni(s) c. Fe(s) + Mn²+ (aq) d. 2 Al(s) + 3 Pb²+ (aq) Ca(s) + Zn²+ (aq) 2 Ag(s) + Ni²+ (aq) Fe²+ (aq) + Mn(s) 2 Al³+ (aq) + 3 Pb(s)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
a Ca2 aq Zns Cas Znaq The halfreactions involved are Zns Znaq 2e E 076 V Caaq 2e Cas E 287 V Since t...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Determine whether each redox reaction occurs spontaneously in the forward direction. a. Ni(s) + Zn+ (aq) b. Ni(s) + Pb+ (aq) c. Al(s) + 3 Ag+ (aq) d. Pb(s) + Mn+ (aq) 2+ Ni+(aq) + Zn(s) Ni+(aq) +...
-
At 298 K, for the reaction 2 PCl 3 (g) + O 2 (g) 2 POCl 3 (l), r H = -620.2 kJ mol -1 and the standard molar entropies, in J mol 1 K 1 , are PCl 3 (g), 311.8; O 2 (g), 205.1; and POCl 3 (l), 222.4....
-
At 298 K, for the reaction 2 H + (aq) + 2 Br - (aq) + 2 NO 2 (g) Br 2 (l) + 2 HNO 2 (aq), r H = -61.6 kJ mol -1 and the standard molar entropies are H + (aq), 0 J mol -1 K -1 ; Br - (aq), 82.4 J...
-
In a bygone day, airlines issued discount tickets to students who would be willing to fly on a particular day, with no notice, at a discounted price, one needed to show proof of being of student. The...
-
As a recently hired internal auditor for the Emerson Department Store (which has approximately 500 employees on its payroll), you are currently reviewing the stores procedures for preparing and...
-
Between 2000 and 2017, real GDP per person grew, on average, 1% per year in the United States. Did this GDP growth benefit all Americans? What does this tell us about the limitations of GDP as a...
-
Assume the tactic of naming a catering director will be implemented. Outline the subject matter to be covered in a training lesson to update the assistant manager about his or her staff party...
-
Suppose you conduct currency carry trade by borrowing $1,000,000 at the start of each year and investing in the New Zealand dollar for one year. One-year interest rates and the exchange rate between...
-
Required information (The following information applies to the questions displayed below. Luke sold a building and the land on which the building sits to his wholly owned corporation, Studemont...
-
An earthmoving project has available one loader with a 6-cy heaped capacity bucket and a larger loader with a 7-cy heaped capacity bucket for filling 45.9-cy heaped capacity off-road trucks. All...
-
Suppose you wanted to cause Ni 2+ ions to come out of solution as solid Ni. Which metal could you use to accomplish this?
-
Determine whether each reaction is a redox reaction. For each redox reaction, identify the oxidizing agent and the reducing agent. a. Al(s) + 3 Ag+ (aq) b. SO3(g) + HO(1) c. Ba(s) + Cl(g) d. Mg(s) +...
-
The IASBs failure to decide on a capital maintenance concept is regrettable as users have no idea as to whether total gains represent income or capital and are therefore unable to identify a...
-
The COVID pandemic has created a crisis for many restaurateurs. The author of one of this week's readings has a suggestion that he thinks could help restaurants survive the crisis. Read the article...
-
Evidence is used to make a decision whenever the decision follows directly from the evidence (Tingling & Brydon, 2010). This is where so many people get it wrong or going by their personal beliefs or...
-
Pick 2 countries, find the price of a Big Mac in each country (if you want to pick another good/service, go ahead), express the price in the local currency, then with the help of exchange rate,...
-
Your task is to educate the public about the role of the Fed in the economy. Role: You are an economic issues reporter for PBS. Audience: Television audience of The Newshour on PBS Situation: Your...
-
Trade Queens Limited is a highly successful FMCG in Zambia. Salient points from the Year-end report indicate the following: Operating profit for the 2022 financial year is up 60% year on year,...
-
Is it possible to have a copper-silver alloy of composition 50 wt% Ag-50 wt% Cu, which, at equilibrium, consists of and phases having mass fractions W = 0.60 and W = 0.40? If so, what will be the...
-
Why should you not model a decision variable as a random variable with a probability distribution?
-
Two hockey players are traveling at velocities of v 1 = 12 m/s and v 2 = -18 m/s when they undergo a head-on collision. After the collision, they grab each other and slide away together with a...
-
Consider an elastic collision in one dimension that involves objects of mass 2.5 kg and 4.5 kg. The larger mass is initially at rest, and the smaller one has an initial velocity of 12 m/s. Find the...
-
Two billiard balls undergo an elastic collision as shown in Figure P7.27. Ball 1 is initially traveling along x with a speed of 10 m/s, and ball 2 is at rest. After the collision, ball 1 moves away...
-
If you made a fixed deposit of $10,000 with an annual interest rate of 3% but the rate of inflation for that year is 3% as well, the calculation of Real Interest Rate would be like this
-
Miller Brothers Hardware paid an annual dividend of $1.80 per share last month. Today, the company announced that future dividends will be increasing by 3.20 percent annually. If you require a 9.5...
-
We know that possessing common stocks represents the corresponding ownership of that share of the companys assets. Suppose an investor buys 1% of equity of a levered firm, then her payoff will be A....

Study smarter with the SolutionInn App


