Each reaction is allowed to come to equilibrium, and then the volume is changed as indicated. Predict
Question:
Each reaction is allowed to come to equilibrium, and then the volume is changed as indicated. Predict the effect (shift right, shift left, or no effect) of the indicated volume change.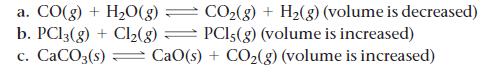
Transcribed Image Text:
CO₂(g) + H₂(g) (volume is decreased) PCI,(g) (volume is increased) a. CO(g) + H₂O(g) - - b. PC13(g) + Cl₂(g) c. CaCO3(s) CaO(s) + CO₂(g) (volume is increased)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (7 reviews)
a When the volume is decreased the reaction will try to offset the change by shifting in the directi...View the full answer

Answered By

Seema kuldeep
although I don't have an experience of teaching in a particular institute, previously I was an expert on Chegg and I have used to teach my batch mates and also my juniors.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. Predict the effect (shift right, shift left, or no effect) of the indicated volume...
-
Each reaction is allowed to come to equilibrium, and then the volume is changed as indicated. Predict the effect (shift right, shift left, or no effect) of the indicated volume change. a. 1(g) 21(g)...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
1. A projectile is launched in a vertical plane, at an angle 0 with initial velocity vo. It must be caught in a frictionless circular tube of radius R in such a way that the trajectory of the...
-
Refer to Exercise 15-3. Suppose the following input prices are provided for each year: Required: 1. Compute the profit-linked productivity measure. By how much did profits increase due to...
-
Based on the work of Coates, Humphreys, and Zhou, it appears that hometown fans are happy to see lopsided wins. Yet at the league level, it appears that competitive balance is crucial. How can we...
-
(This problem relates to P3-3). You have just been hired as a stock analyst for a large stock brokerage company. Your first assignment is to analyze the performance of Gidley Electronics. Presented...
-
Sherman Company employs 400 production, maintenance, and janitorial workers in eight separate departments. In addition to supervising operation, the supervisors of the departments are responsible for...
-
What is the yield to maturity of a 10 year $1000 bond with a 6.8% semi-annual coupon that is currently trading for $1040 answer as a % rounded to two digits after the decimal: xxx%)
-
Stewart Enterprises has the following investments, all purchased prior to 2024: 1. Bee Company 5% bonds, purchased at face value, with an amortized cost of $4,000,000, and classified as...
-
This reaction is exothermic. Predict the effect (shift right, shift left, or no effect) of increasing and decreasing the reaction temperature. How does the value of the equilibrium constant depend on...
-
Consider this reaction at equilibrium: Predict whether the reaction will shift left, shift right, or remain unchanged after each disturbance. a. C is added to the reaction mixture. b. H 2 O is...
-
Figure shows part of the experimental arrangement in which antiprotons were discovered in the 1950s. A beam of 6.2 GeV protons emerged from a particle accelerator and collided with nuclei in a copper...
-
Computing Unit Cost Comacho Chemical Co. recorded costs for the month of $18,900 for materials, $44,100 for labor, and $26.250 for factory overhead. There was no beginning work in process, 8.000...
-
Problem Joe Fox is the manager of the Gear division and plans to submit a proposal for an expanded production area. Joe has projected various conditions for revenues and expense for the expansion,...
-
Significant Values. In Exercises 9-12, use the range rule of thumb to identify (a) the values that are significantly low, (b) the values that are significantly high, and (c) the values that are...
-
A study requires a group of 3 people to be interviewed from an organization with 50 members. How many groups of 3 are possible?
-
Univex is a calendar year, accrual basis retail business. Its financial statements provide the following information for the year: Revenues from sales of goods$783,200Cost of goods sold...
-
Suppose at time t = 0, we are given four zero-coupon bond prices {B1, B2, B3, B4} that mature at times t = 1, 2, 3, 4. This forms the term structure of interest rates. We also have one-period forward...
-
What is EBIT/eps analysis? What information does it provide managers?
-
A system is being designed to carry 500 gal/min of ethylene glycol at 77F at a maximum velocity of 10.0 ft/s. Specify the smallest standard Schedule 40 steel pipe to meet this condition. Then, for...
-
The range of Reynolds numbers between 2000 and 4000 is described as the critical region because it is not possible to predict whether the flow is laminar or turbulent. One should avoid operation of...
-
The water line described in Problem 8.22 was a cold water distribution line. At another point in the system, the same-size tube delivers water at 180F. Compute the range of volume flow rates for...
-
ABC Corp currently has a debt to enterprise value ratio of 51%. The firm's cost of equity is 8.4% and its cost of debt is 4%. Assuming perfect markets, calculate the unlevered cost of capital for ABC...
-
Rebound Airlines was hurt in the recession, with its stock falling to $10 from $50. The stock has started to recover and has just crossed its 50-day moving average at $20. The 200-day moving average...
-
Biotech Corp has no sales or earnings but is working on a COVID cure. They also have some good prospects for other drugs that could potentially be blockbusters. The stock started the year at $30 and...

Study smarter with the SolutionInn App


