Find the equilibrium constant at 298 K for the reaction. The K f for [Cu(NH 3 )
Question:
Find the equilibrium constant at 298 K for the reaction.![]()
The Kf for [Cu(NH3)2]+ = 6.3 * 1010 and the rest of the data needed are in Appendix II.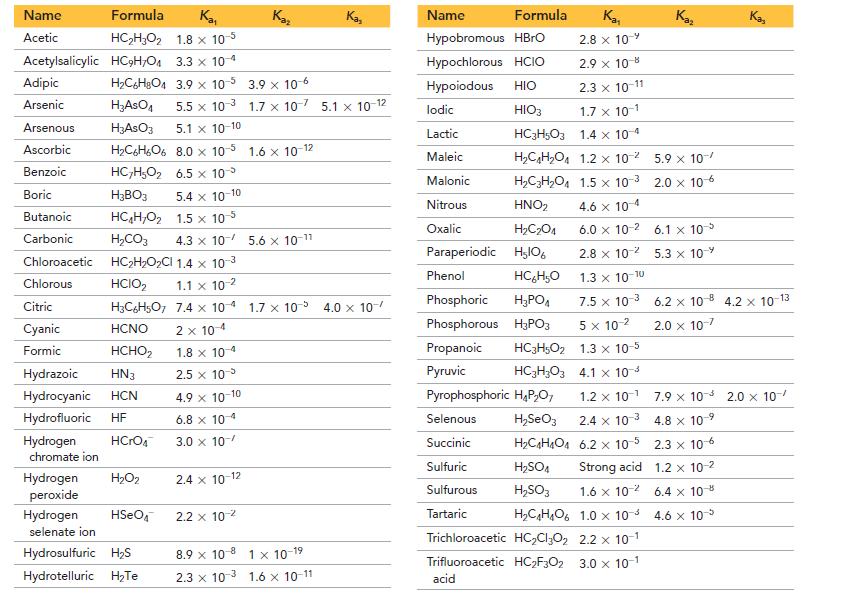
Transcribed Image Text:
2 [Cu(NH3)2](aq) = [Cu(NH3)4]²+ (aq) + Cu(s)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (2 reviews)
To find the equilibrium constant for the reaction 2 CuNH32aq CuNH34 aq Cus at 298 K we ca...View the full answer

Answered By

ANDREW KIPRUTO
Academic Writing Expert
I have over 7 years of research and application experience. I am trained and licensed to provide expertise in IT information, computer sciences related topics and other units like chemistry, Business, law, biology, biochemistry, and genetics. I'm a network and IT admin with +8 years of experience in all kind of environments.
I can help you in the following areas:
Networking
- Ethernet, Wireless Airmax and 802.11, fiber networks on GPON/GEPON and WDM
- Protocols and IP Services: VLANs, LACP, ACLs, VPNs, OSPF, BGP, RADIUS, PPPoE, DNS, Proxies, SNMP
- Vendors: MikroTik, Ubiquiti, Cisco, Juniper, HP, Dell, DrayTek, SMC, Zyxel, Furukawa Electric, and many more
- Monitoring Systems: PRTG, Zabbix, Whatsup Gold, TheDude, RRDtoo
Always available for new projects! Contact me for any inquiries
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
For the following exercises, use the vertical line test to determine which graphs show relations that are function x m II
-
Find the equilibrium constant at 298 K for the reaction. The K f for [Cu(CN) 2 ]- = 1.0 * 10 24 and the rest of the data needed are in Appendix II. [Ag(CN)] (aq) + Cu(s) [Cu(CN)] (aq) + Ag(s)
-
Muharraq Co. paid the following different costs in 2020: 1. $2,000,000 to acquire a machine to be used in the R&D projects. The machine has a 4- year useful life (Muharraq Co. started using the...
-
If f(x) = 5x + 1, find and simplify each of the following in Problem. f(f( -1))
-
Jigar Tech, Inc., is authorized to issue $1,800,000 in bonds on June 1. The bonds carry a face interest rate of 9 percent, which is to be paid on June 1 and December 1. Prepare entries in journal...
-
Consider again the conditions of Exercise 15, but suppose now that both parameters x0 and are unknown. Determine the M.L.E.s of x0 and . In Exercise 15 Suppose that X1, . . . , Xn form a random...
-
Colgate-Palmolive sells its products in many countries throughout the world. How would you expect its marketing strategy to differ in various countries, compared with that in the United States?...
-
Some rearview mirrors produce images of cars behind you that are smaller that they would be if the mirror were flat. Are the mirrors concave or convex? What is the mirrors radius of curvature if cars...
-
Fudzis Ltd.s income before interest and tax is GHS 900,000. The firm currently employs a debt of GHS 300,000 at a cost of 10%. The weighted average cost of capital of the firm is 12%. What is the...
-
Tin exists in two allotropic forms. Gray tin has a diamond structure, and white tin has a close-packed structure. Predict which allotrope is (a) Denser, (b) A conductor of electricity. Predict the...
-
Hydrogen can be in both the octahedral and tetrahedral holes for lanthanum. Determine the percentage of the holes that are filled if the formula is LaH 2.76 .
-
Which of the following statements about equivalence partitioning as a testdata design method is false? a. One test data element should be selected that falls within each equivalence class, and one...
-
What makes a set of objects a vector space? You will no doubt want to refer to notes and the text, but I'd like you to summarize it for starters. If you have identified a vector space, for example...
-
Walla Walla Company is in its planning stage for next year. Walla Walla expects a big Quarter 3 and is creating a production budget to determine if it needs to hire more employees. Walla Walla knows...
-
Task: P9 P9a P9b P9c P9d Describe the principles and applications of electromagnetic induction Describe using a series of bullet point statements, how transformers work and how their operation...
-
Based on the given information, analyse company's financial health and provide future projections. Unit FY16 FY17 FY18 FY19 Sales Rs. Cr 134 245 371 1,159 PAT Rs. Cr (281) (585) (78) (571) Assets Rs....
-
OBJECTIVE QUESTIONS 1. In each of the following only one statement/item is correct. State which. (i) Financial Accounting helps in (a) ascertaining the financial position of the concerned firm, (b)...
-
Refer to P3-4. Kaylee James, a connoisseur of fine chocolate, opened Kaylee's Sweets in Collegetown on February 1. The shop specializes in a selection of gourmet chocolate candies and a line of...
-
A certain Christmas tree ornament is a silver sphere having a diameter of 8.50 cm. Determine an object location for which the size of the reflected image is three-fourths the size of the object. Use...
-
Two point particles of charge Q 1 = 45 C and Q 2 = 85 C are found to have a potential energy of 40 J. What is the distance between the charges?
-
Two particles with Q 1 = 45 C and Q 2 = 85 C are initially separated by a distance of 2.5 m and then brought closer together so that the final separation is 1.5 m. What is the change in the electric...
-
The nucleus of a helium atom contains two protons. In a simple model of this nucleus, the protons are viewed as point particles separated by 1.0 fm (1.0 10 -15 m). What is the electric potential...
-
Chapter o Homew ebook 50,000-unit production quantity: $ 227,049 7 70,000-unit production quantity: $ 66,751 d. In addition to mean profit, what other factors should FTC consider in determining a...
-
Diamond makes downhill ski equipment. Assume that comic has offered to produce ski poles for Diamond for $20 per pair Diamond needs 200,000 pairs of poles per period Diamond can only avoid 5150,000...
-
17? Which of the following statement is true Select one: a. All evidence must have the same level of reliability b. All evidence must have the same level of persuasiveness C. All are false d....

Study smarter with the SolutionInn App


