For each compound, would you expect greater solubility in water or in hexane? Indicate the kinds of
Question:
For each compound, would you expect greater solubility in water or in hexane? Indicate the kinds of intermolecular forces that would occur between the solute and the solvent in which the molecule is most soluble.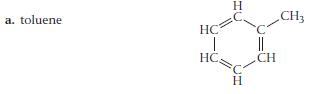
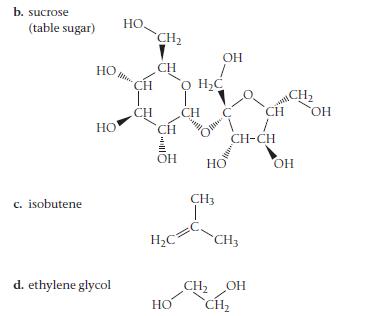
Transcribed Image Text:
a. toluene. HC | HC Н Н || CH CH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
a Toluene Polarity Nonpolar Expected solubility Greater solubility in hexane Intermolecular forces D...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
For each compound, would you expect greater solubility in water or in hexane? Indicate the kinds of intermolecular forces that occur between the solute and the solvent in which the molecule is most...
-
1. Look up and draw the structures for each of the following molecules: (a) glucose (b) naphthalene (c) dimethyl ether (d) alanine For each molecule, would you expect greater solubility in water or...
-
Pick an appropriate solvent from Table 14.3 to dissolve each substance. State the kind of intermolecular forces that would occur between the solute and solvent in each case. a. Isopropyl alcohol...
-
Discuss the impact of commissions in organizations growth?
-
Jerry Goff, president of Harmony Electronics, was concerned about the end-of-the-year marketing report that he had just received. According to Emily Hagood, marketing manager, a price decrease for...
-
True or false? It is sometimes said that an economist is a person who knows the price of everything but the value of nothing. Why?
-
Ranking of capital budgeting projects, alternative selection methods, capital rationing. (CMA, adapted) Brendan Rogers, division president of Wildwood Manufacturing, is prepar ing the 2008 capital...
-
Given the following production schedule, compute the available-to-promisequantities. WEEK 4 Model B MPS 20 20 20 BI 20 20 20 20 Committed Customer Orders10 1010 1010 ATP:D 10
-
Please Help, Thank You! Smart Company prepared its annual financial statements dated December 31,2020 . The company applies the FIFO inventory costing method; however, the company neglected to apply...
-
Wayland Custom Woodworking is a firm that manufactures custom cabinets and woodwork for business and residential customers. Students will have the opportunity to establish payroll records and to...
-
When ammonium chloride (NH 4 Cl) is dissolved in water, the solution becomes colder. a. Is the dissolution of ammonium chloride endothermic or exothermic? b. What can you conclude about the relative...
-
Which molecule would you expect to be more soluble in water: CCl 4 or CH 2 Cl 2 ?
-
What is a distillation boundary? Why is it important?
-
A corporation issues 13 %, 15-year bonds with a par value of $570,000 and semiannual interest payments. On the issue date, the annual market rate for these bonds is 11%, which implies a selling price...
-
You are working in the C++ Care Unit. As the patients come in you need to first calculate their BMI with your BMI Machine before they make the appointment. Your scale gives weight in KG but you can...
-
University Printers has two service departments (Maintenance and Personnel) and two operating departments (Printing and Developing). Management has decided to allocate maintenance costs on the basis...
-
In 2026, Blossom Company purchased the net assets of Ayayai Corporation for $2226400. On the date of the transaction, Ayayai had $607200 of liabilities. The fair value of Ayayai's assets when...
-
Financial statement data for the years ending December 31, 20Y3 and 20Y2, for Lawson Company follow: Sales Total assets: Beginning of year End of year 20Y3 $1,400,000 610,000 790,000 20Y2 $1,026,000...
-
Determine the indices for the directions shown in the following cubic unit cell: +2
-
What is beacon marketing? What are digital wallets?
-
Imagine a skydiver who waits a long time before opening her parachute. For simplicity, assume she moves along a straight line. (a) In what direction is the skydiver moving (what is the direction of...
-
In our discussion of the block and tackle in Figure 3.23, we claimed that when the right end of the string is lifted through a distance L, the body of the pulley is lifted a distance L/2. Give a...
-
An astronaut measures her mass and her weight on Earth and again when she reaches the Moon. Which one of these quantities changes as a result of this trip and which one does not? Explain.
-
The yield to maturity is not the compound annual rate of return earned on a debt security purchased on a given day and held to maturity. true or false
-
If 1500 is deposited at the end of each quarter in an account that earns 4% compounded quarterly, after how many quarters will the account contain 60,000? (round your answer up to the nearest quarter)
-
With a 35 percent marginal tax rate, would a tax-free yield of 6.1 percent or a taxable yield of 10 percent give you a better return on your savings?

Study smarter with the SolutionInn App


