For each compound, would you expect greater solubility in water or in hexane? Indicate the kinds of
Question:
For each compound, would you expect greater solubility in water or in hexane? Indicate the kinds of intermolecular forces that occur between the solute and the solvent in which the molecule is most soluble.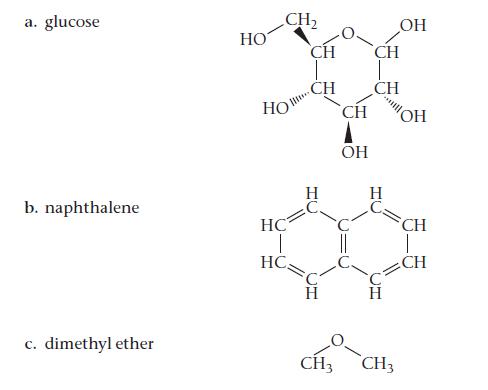
Transcribed Image Text:
a. glucose b. naphthalene c. dimethyl ether HO CH₂ НО HC 1 HC CH CH LC TI 2) Н CH ОН CH CH H OH CH3 CH3 OH CH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 62% (8 reviews)
a Water dispersion d...View the full answer

Answered By

Branice Buyengo Ajevi
I have been teaching for the last 5 years which has strengthened my interaction with students of different level.
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
For each compound, would you expect greater solubility in water or in hexane? Indicate the kinds of intermolecular forces that would occur between the solute and the solvent in which the molecule is...
-
1. Look up and draw the structures for each of the following molecules: (a) glucose (b) naphthalene (c) dimethyl ether (d) alanine For each molecule, would you expect greater solubility in water or...
-
Pick an appropriate solvent from Table 14.3 to dissolve each substance. State the kind of intermolecular forces that would occur between the solute and solvent in each case. a. Isopropyl alcohol...
-
Please show your work D To be a valid route, it must consist of a sequence of valid move. Each valid move is either going to the right for one block or going down for 1 block. T d) How many valid...
-
Daspart, Inc. supplies carburetors for a large automobile manufacturing company. The auto company has recently requested that Daspart decrease its delivery time. Daspart made a commitment to reduce...
-
The figure below shows the budget deficit function for a country. a. Explain why the budget deficit function is downward sloping. b. If the government increases its level of purchases \((G)\), what...
-
Robotics capital project, inflation, income taxes. Rustbelt, Inc., purchases second-hand pipeline equipment and rehabilitates it for resale. Rustbelt has experienced many indus trial accidents...
-
Medeiros Manufacturing, Inc. has a manufacturing machine that needs attention. The company is considering two options. Option 1 is to refurbish the current machine at a cost of $ 1,000,000. If...
-
Jpeliai Unuel Smooth Move Company manufactures professional paperweights and has been approached by a new customer with an offer to purchase 15,000 units at a per-unit price of $7.00. The new...
-
A work sheet for Juanita's Consulting is shown on the following page. There were no additional investments made by the owner during the month. REQUIRED 1. Prepare an income statement. 2. Prepare a...
-
Which molecule would you expect to be more soluble in water: CCl 4 or CH 2 Cl 2 ?
-
Which molecule would you expect to be more soluble in water: CH 3 CH 2 CH 2 OH or HOCH 2 CH 2 CH 2 OH?
-
Visit www.distrowatch.com. Click on one of the top five listed Linux distributions (like Mint, Ubuntu, Debian, Fedora, or OpenSUSE). Click on the Screenshots link for that distribution. List some...
-
The concentration of two medicines Prinivil and Zestril in the bloodstream x hours after being injected are: Prinivil: f(x) = xe* and Zestril: h(x) = xe x>0 When (if at all) does Prinivil disperse...
-
Over a three-day period, Kennedy's Restaurant had the following information. Thursday Friday Saturday Total Revenue $1,800 $3,300 $4,700 Number of Guests 70 91 110 Servers 5 8 13 Do not enter dollar...
-
Warnerwoods Company uses a perpetual inventory system. It entered into the following purchases and sales transactions for March. Date Units Acquired at Cost: March 1 Activities Beginning inventory...
-
Newly formed S&J Iron Corporation has 196,000 shares of $6 par common stock authorized. On March 1, Year 1, S&J Iron issued 10,000 shares of the stock for $11 per share. On May 2, the company issued...
-
After assessing the credit risk characteristics of its accounts receivable, a company determined that the length of time a receivable was outstanding was the most appropriate risk characteristic to...
-
Show for the body-centered cubic crystal structure that the unit cell edge length a and the atomic radius R are related through a = 4R/3.
-
An educational researcher devised a wooden toy assembly project to test learning in 6-year-olds. The time in seconds to assemble the project was noted, and the toy was disassembled out of the childs...
-
A motivated mule can accelerate an empty cart of mass m = 180 kg from rest to 5.0 m/s in 10 s. If the cart is loaded with 540 kg of wood, how long will it take the mule to get the cart to 5.0 m/s?...
-
A person has a weight of 500 N. What is her mass?
-
A book of mass 3.0 kg sits at rest on a horizontal table. What is the magnitude of the normal force exerted by the table on the book?
-
A total of 2,000 units of Product A are produced from a joint process. Product A can be sold at the split-off point for $16 per unit, or it can be processed further for an additional total cost of...
-
How has COVID - 19 affected the job market in Canada Summary of all research conducted with a minimum of 3 credible sources? An activity you will be using to engage the audience and enforce the...
-
please as soon as can i will rate the thumps up IF THE BANK GIVES YOU 5% INTEREST ON YOUR SAVING ACCOUNT AND INFLATION IS 3% WHAT IS YOU Select one: O a. CANNOT BE CALCULATED b. 1.94% O c. 2% O d....

Study smarter with the SolutionInn App


