For each molecular geometry, list the number of total electron groups, the number of bonding groups, and
Question:
For each molecular geometry, list the number of total electron groups, the number of bonding groups, and the number of lone pairs on the central atom.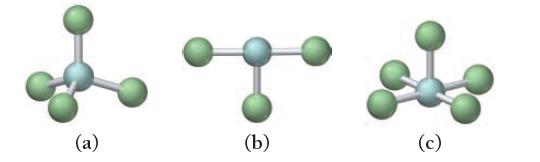
Transcribed Image Text:
(a) (b) (c)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
a 4 e groups b 5 e groups ...View the full answer

Answered By

Srilekha Talluri
I am a final year Computer science student at Bits Hyderabad. I have recently completed my internship at Amazon. I worked as a SDE Intern with Seller central team of Amazon. I have also done an internship with Human Resource department of Telangana Govt Secretariat. Due to these exposure i had the opportunity of putting my subject into action and make an impact.
I have also done good projects in my Discipline and worked as teaching assistant for few important Computer science courses like Data Structures and Algorithms, Discrete Mathematics. As a computer science student, I have completed lot of courses like Cryptography, Graph theory, Design and Analysis of Algorithms etc.
My teaching experience has been awesome throughout .I have had many occasions to experience teaching. I have been fortunate enough to teach underprivileged children as a part of a NGO on college. I taught them the essential aspects of writing, mathematics,science etc. They were able to excel in the board exams they undertook that year and got themselves admitted to XII class.
The experience was wonderful and it caught me by surprise that many of the students never realize their true potential and just need a little external help to overcome huge barriers in academics.
Working as a teaching Assistant for disciplinary courses also helped me to gain good knowledge and tutoring experience
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
For each molecular geometry, list the number of total electron groups, the number of bonding groups, and the number of lone pairs on the central atom. (a) (b) (c)
-
Describe a hybridization scheme for the central S atom in the molecule SF 4 that is consistent with the geometric shape pictured in Table 10.1. Which orbitals of the S atom are involved in overlaps,...
-
Describe a hybridization scheme for the central Cl atom in the molecule ClF 3 that is consistent with the geometric shape pictured in Table 10.1. Which orbitals of the Cl atom are involved in...
-
Growth Strategy A. Organic Growth This section describes how JB Hi-Fi Australia can take advantage of various organic growth strategies, including new product development, market development and...
-
Sunk costs are easy to spottheyre simply the fixed costs associated with a decision. Do you agree? Explain.
-
Smyth Inc. has decided to do a scenario analysis for the year 2022. Their pessimistic prediction for sales is $360,000, the most likely amount of sale is $450,000, and the optimistic sales prediction...
-
For items that it sells itself, compute AllRoad Parts' cost of using FBA to process: a. An order of 10 sets of bicycle pedals weighing a total of 25 pounds. b. An order of a single set of bicycle...
-
Cable Corporation, which operates a fleet of motorized trolley cars in a resort city, is undergoing a complete liquidation. John, who owns 80% of the Cable stock, plans to continue the business in...
-
Two risky assets with expected rates of return and have identical variances and a known correlation coefficient . There is also a risk-free asset with a rate of return . Using mean-variance portfolio...
-
Assume an organizations current service level on order fill is as follows: Current order fill = 70% Number of orders per year = 10,000 Percent of unfilled orders back-ordered = 85% Percent of...
-
Determine the electron geometry, molecular geometry, and idealized bond angles for each molecule. In which cases do you expect deviations from the idealized bond angle? a. PF 3 b. SBr 2 c. CHCl 3 ...
-
A molecule with the formula AB 3 has a trigonal planar geometry. How many electron groups are on the central atom?
-
Bill Christensen, the production manager, was grumbling about the new quality cost system the plant controller wanted to put into place. If we start trying to track every bit of spoiled material,...
-
Which one of the following therapists' approaches has been integrated into several other therapies in the West? 1. Naikan therapy 2. Morita therapy 3. mindfulness meditation
-
What does this scatter plot tell us? Check ALL below that are true from the Scatter Plot. Y is cumulative total barrels and x is number of active wells. 1. If there were 550 active wells, we would...
-
Millie runs a small company that makes customised notebooks with personalised details on the cover and inserts. You promote your product as a great gift idea, and your holiday orders break your...
-
Excel ACC 311 Project Two Workbook Template - View-only Search (Alt + Q) File Home Insert Draw Page Layout Formulas Data Review View Help 12 B A ... ab Ev F10 1 2 3 4 5 6 fx A B Posey's Pet Emporium...
-
Imagine that you are the change manager for acompany that does business entirely via the Internet. The head development engineercalls to indicate he wants to make a small change to one of the...
-
Briefly describe the Meissner effect.
-
List four items of financial information you consider to be important to a manager of a business that has been operating for a year.
-
The A-36 steel plate has a thickness of 12 mm. If Ï allow = 150 MPa, determine the maximum axial load P that it can support. Calculate its elongation, neglecting the effect of the fillets. r= 30...
-
If the allowable normal stress for the bar is Ï allow = 120 MPa, determine the maximum axial force P that can be applied to the bar. 5 mm 40 mm 20 mm r = 10 mm 20 mm
-
The assembly consists of two posts AB and CD each made from material 1 having a modulus of elasticity of E 1 and a cross-sectional area A1, and a central post EF made from material 2 having a modulus...
-
Williams Pharmaceuticals issued 150 million shares of its $1 par common stock at $15 per share in 2014. They had the following transactions during 2014: Jun. 27: Williams Pharmaceuticals reacquired...
-
Adjusting entries ABC. Co purchased 600 of office supplies on account on October 15th. On December 31st, 150 of office supplies remain on hand. Prepare the journal entries to record the October 15th...
-
Calculate the present value of cash flows, 1500 in the year 1 then grows at 2% every year, using 10% discount rate. Round and write up to two decimals (e.g., 100.00). No characters including comma...

Study smarter with the SolutionInn App


