Gaseous methane reacts with oxygen to form carbon dioxide and water vapor. Write a balanced equation for
Question:
Gaseous methane reacts with oxygen to form carbon dioxide and water vapor. Write a balanced equation for the combustion reaction and determine which mixture has neither reactant in excess.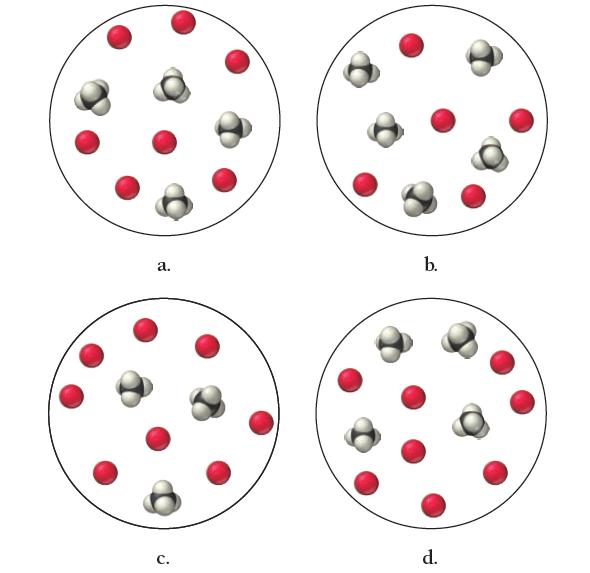
Transcribed Image Text:
a. C. b. d.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
The balanced equation for the combustion reaction of methane and oxygen is CH4 2 O2 CO2 2 H2O This e...View the full answer

Answered By

Pushpinder Singh
Currently, I am PhD scholar with Indian Statistical problem, working in applied statistics and real life data problems. I have done several projects in Statistics especially Time Series data analysis, Regression Techniques.
I am Master in Statistics from Indian Institute of Technology, Kanpur.
I have been teaching students for various University entrance exams and passing grades in Graduation and Post-Graduation.I have expertise in solving problems in Statistics for more than 2 years now.I am a subject expert in Statistics with Assignmentpedia.com.
4.40+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A 16.0-g sample of methane (CH 4 ) reacts with 64.0 g of oxygen gas in a container fitted with a piston (at 1.00 atm and 425 K). Methane can react with oxygen to form carbon dioxide and water vapor...
-
QUESTION 1 When propane undergoes complete combustion, the products are carbon dioxide and water.? ? ? ? __ C 3 H 8 (g) + __ O 2 (g) ? __ CO 2 (g) + __ H 2 O(g)What are the respective coefficients...
-
A 1.604-g sample of methane (CH4) gas and 6.400 g of oxygen gas are sealed in a 2.50- L vessel at 411oC and are allowed to reach equilibrium. Methane can react with oxygen to form gaseous carbon...
-
You are the manager of a fast food restaurant. Part of your job is to report to the boss at the end of the day which special is selling best. Use your vast knowledge of descriptive statistics and...
-
If the Crash Davis Driving School has a 14.2 percent ROE and a 25 percent payout ratio, what is its sustainable growth rate?
-
The demand for electric power is usually much higher during the day than it is at night, and utility companies often sell power at night at much lower prices to encourage consumers to use the...
-
Identify and briefly compare the two leading stock exchanges in the United States today. AppendixLO1
-
1. Draw a diagram of the drop process. How long should it take to empty 300 silver dollar slot machines? 2. Draw a diagram of the hard count process. How long should this process take to complete for...
-
Bowie Sporting Goods manufactures sleeping bags. The manufacturing standards per sleeping bag, based on 5,000 sleeping bags per month, are as follows: Direct material of 4.50 yards at $5.60 per yard...
-
Alabama Atlantic is a lumber company that has three sources of wood and five markets to be supplied. The annual availability of wood at sources 1, 2, and 3 is 15, 20, and 15 million board feet,...
-
The reaction of NH 3 and O 2 forms NO and water. The NO can be used to convert P 4 to P 4 O 6 , forming N 2 in the process. The P 4 O 6 can be treated with water to form H 3 PO 3 , which forms PH 3...
-
The combustion of liquid ethanol (C 2 H 5 OH) produces carbon dioxide and water. After 4.62 mL of ethanol (density = 0.789 g/mL) is allowed to burn in the presence of 15.55 g of oxygen gas, 3.72 mL...
-
Cool Rays Ltd. is considering dropping its Tinted Glass product line. The Tinted Lens product line income statement for the last year was as follows: The company has a total of 3 product lines. Only...
-
Lab 1-3 Data Analytics in Auditing The purpose of this lab is to help you identify relevant questions that may be answered using data analytics in auditing. Let's evaluate how we might use master and...
-
Geography Majors The data listed below are estimated incomes (dollars) of students who graduated from the University of North Carolina (UNC) after majoring in geography. The data are based on...
-
Reproduced below from Farthington Supply's accounting records is the accounts receivable subledger along with selected general ledger accounts. Dec. 31/22 Balance Credit sales in 2023 General Ledger...
-
Listed below are pulse rates (beats per minute) from samples of adult males and females. Does there appear to be a difference? Find the coefficient of variation for each of the two samples; then...
-
When the price of an item goes down, people buy more of it because at a lower price Options: -some new people may be willing to enter the market. -the item is more valuable. -the marginal cost is...
-
A cell is diploid and contains three chromosomes per set. Draw the arrangement of chromosomes during metaphase of mitosis and metaphase of meiosis I and II. In your drawing, make one set dark and the...
-
Record the following selected transactions for March in a two-column journal, identifying each entry by letter: (a) Received $10,000 from Shirley Knowles, owner. (b) Purchased equipment for $35,000,...
-
Calculate S for the reaction 3H 2 (g) + N 2 (g) 2NH 3 (g) at 725 K. Omit terms in the temperature-dependent heat capacities higher than T 2 /K 2 .
-
Using toluene and acetylene as your only sources of carbon atoms, show how you would prepare the following compound.
-
Using the expression dS = C p /T dt VdP, calculate the decrease in temperature that occurs if 2.25 moles of water at 310. K and 1650. bar is brought to a final pressure of 1.30 bar in a reversible...
-
Analysis & Summary Based on your search results, conduct an analysis of the requirement to qualify for the position/career in which you are interested. Then follow the instructions, below- Accounting...
-
Your Corporation uses activity-based costing to determine product costs. The company has provided the following data concerning its activity-based costing system: Activity Cost Pools(and Activity...
-
Maryville Inc. incurred the following costs during August: Raw materials used Direct labor Manufacturing overhead, actual Selling expenses Administrative expenses Interest expense $ 39,300 68,000...

Study smarter with the SolutionInn App


