Have each group member look up the K sp for a different compound. Calculate the molar solubility.
Question:
Have each group member look up the Ksp for a different compound.
Calculate the molar solubility. Do the numerical values suggest that the compound is soluble or insoluble?
Compare answers with the solubility rules from Chapter 5, and have each group member present his or her findings to the group.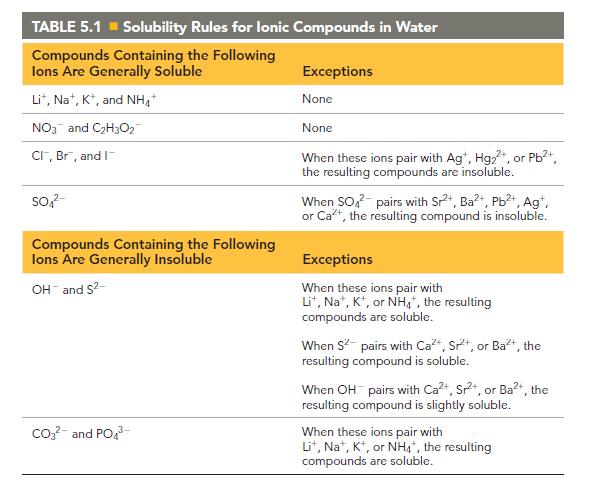
Transcribed Image Text:
TABLE 5.1 Solubility Rules for lonic Compounds in Water Compounds Containing the Following lons Are Generally Soluble Lit, Na+, K+, and NH NO3 and C₂H3O2 CI, Br, and I SO4²- Compounds Containing the Following lons Are Generally Insoluble OH and S²- CO3²- and PO4³- Exceptions None None When these ions pair with Agt, Hg₂+, or Pb²+, the resulting compounds are insoluble. When SO² pairs with Sr²+, Ba²+, Pb²+, Ag+, or Ca²+, the resulting compound is insoluble. Exceptions When these ions pair with Lit, Na+, K+, or NH4+, the resulting compounds are soluble. When S² pairs with Ca+, Sr, or Ba²+, the resulting compound is soluble. When OH pairs with Ca²+, Sr²+, or Ba²+, the resulting compound is slightly soluble. When these ions pair with Lit, Na, K, or NH4*, the resulting compounds are soluble.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (7 reviews)
Sure lets go through an example Lets say each member of the group is looking up the Ksp solubility product constant for a different compound Ill use a ...View the full answer

Answered By

GERALD KAMAU
non-plagiarism work, timely work and A++ work
4.40+
6+ Reviews
11+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Review the solubility rules. Without referring back to the rules, have each group member list two ionic compounds that are expected to be soluble and two that are expected to be insoluble. Include at...
-
Choose a row of the transition metals in the periodic table. Have each group member look up and graph (where appropriate) a separate trend for the elements in that row, choosing from electron...
-
Assign each element in the first row of the transition metals, scandium through zinc, to a group member. Have each group member look up on the Internet or in a reputable chemistry reference book the...
-
MVP Company issued a callable bond. The bond is a 7% semiannual coupon bond currently priced at 102 that has a remaining time to maturity of seven years. The bond is callable beginning the end of...
-
Smith Accounting Services is considering a special order that it received from one of its corporate clients. The special order calls for Smith to prepare the individual tax returns of the...
-
A timber company used a physical performance test to assess candidates for entry-level positions handling lumber and cutting wood. The test was developed by industrial psychologists. In its original...
-
How do firms enhance postpurchase satisfaction and reduce cognitive dissonance?
-
The time needed for performing a machining operation is to be investigated. Historically, the process has had a standard deviation equal to .146 minute. The means (in minutes) of 39 samples of n = 6...
-
ney ba Beppert NE fice yo When answering the questions being asked, please be sure that you focus on interpreting the information, as that is the real assignment here. Don't just list what is...
-
A base is known to be one of the three listed in the table. You are given a sample of the base and asked to identify it. To do so, you dissolve 0.30 g of the base in enough water to make 25.0 mL of...
-
With group members acting as atoms or ions, act out the reaction that occurs when HCl is added to a buffer solution composed of HC 2 H 3 O 2 and NaC 2 H 3 O 2 . Write out a script for a narrator that...
-
Can real images be projected on a screen? Can virtual images? Can either be photographed? Discuss carefully.
-
Dr. Kovaleski is interested in examining whether quantity of sleep impacts problem solving ability. To test problem solving ability, the research team gave participants a puzzle and measured how long...
-
Can you please help me fill out the spreadsheet? Idexo Corporation is a privately held designer and manufacturer of licensed college apparel in Cincinnati, Ohio. In late 2020, after several years of...
-
CHECK FIGURE: Adjusted book balance = $2,837.06 Mae Telford, the controller of the Baylor Company, provided the following information: Bank statement balance Add: Baylor Company Bank Reconciliation...
-
Read the Scenario Congratulations, you are now the Police Chief in Anytown, USA. A city with 30,000 residents and you are responsible to provide 24 hour a day police coverage. You have a total of 45...
-
Here are summary statistics for randomly selected weights of newborn girls: n = 36, x = 3180.6 g, s = 700.5 g. Use a confidence level of 99% to complete parts (a) through (d) below. a. Identify the...
-
Refer to the Costello Music Company problem in exercise 49. a. Using time series decomposition, compute the seasonal indexes for the four quarters. b. When does Costello Music experience the largest...
-
Explain what is meant by vicarious liability and when it is available?
-
A sound wave in an unknown gas has a frequency of 440 Hz and a wavelength of 2.2 m. What type of gas might it be? Use Table 13.1. TABLE 13.1 Speed of Sound in Some Common Materials Speed of Sound...
-
A battleship is using sonar (reflected underwater sound signals) to detect the presence of nearby submarines. It is found that a sonar reflection has a round-trip travel time (from the battleship to...
-
In this chapter, we discussed the Doppler effect that occurs when a source of sound moves directly toward or away from a listener. Do you think there will be a Doppler shift if the velocity of the...
-
1,600 Balance Sheet The following is a list (in random order) of KIP International Products Company's December 31, 2019, balance sheet accounts: Additional Paid-In Capital on Preferred Stock $2,000...
-
Question 3 4 pts 9 x + 3 x 9 if x 0 Find a) lim f(x), b) lim, f(x), C), lim , f(x) if they exist. 3 Edit View Insert Format Tools Table : 12pt M Paragraph B IV A2 Tv
-
Mr. Geoffrey Guo had a variety of transactions during the 2019 year. Determine the total taxable capital gains included in Mr. Guo's division B income. The transactions included: 1. On January 1,...

Study smarter with the SolutionInn App


