How many liters of a 0.125 M NaOH solution contain 0.255 mol of NaOH? SORT You are
Question:
How many liters of a 0.125 M NaOH solution contain 0.255 mol of NaOH?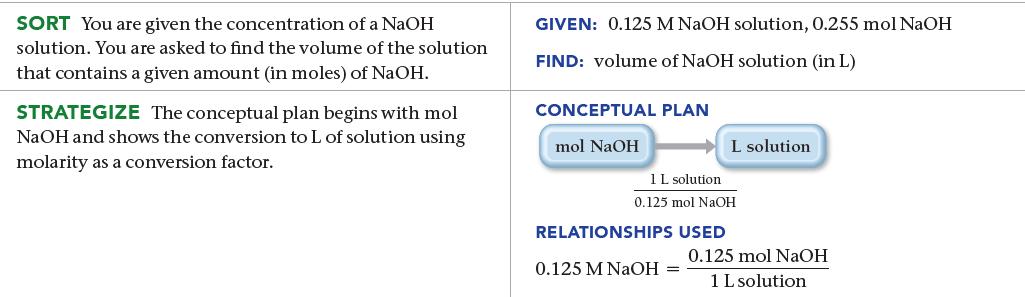
Transcribed Image Text:
SORT You are given the concentration of a NaOH solution. You are asked to find the volume of the solution that contains a given amount (in moles) of NaOH. STRATEGIZE The conceptual plan begins with mol NaOH and shows the conversion to L of solution using molarity as a conversion factor. GIVEN: 0.125 M NaOH solution, 0.255 mol NaOH FIND: volume of NaOH solution (in L) CONCEPTUAL PLAN mol NaOH L solution 1 L solution. 0.125 mol NaOH RELATIONSHIPS USED 0.125 M NaOH = 0.125 mol NaOH 1 L solution
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
0255 mol NaOH X 1 L solution 0125 mol NaOH 204 L ...View the full answer

Answered By

Ashish Jaiswal
I have completed B.Sc in mathematics and Master in Computer Science.
4.90+
20+ Reviews
39+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
-1 Reverse osmosis membrane filtration is to be used to treat brackish (saline) groundwater to supply drinking water for a NSW regional city. Feed water will be provided at a flow rate of 25,000...
-
ABC Co. reported net income for the current year 2018 at P10,000,000 before taxes. Included in the determination of the said net income were: Fines, penalties and surcharges Life insurance expense...
-
How many liters of each of 0.5% (w/v) saline solution and 2% (w/v) to produce 10 L of normal saline? Remember, normal saline is 0.9% (w/v) saline.
-
Nancy has active modified adjusted gross income before passive losses of $75,000. She has a loss of $5,000 on a rental property she actively manages. How much of the loss is she allowed to take...
-
For the most recent year, Fame, Inc., had sales of $378,000, cost of goods sold of $95,400, depreciation expense of $47,000, and additions to retained earnings of $48,750. The firm currently has...
-
Consider the following economy: Assume the SRAS is horizontal at the current price level. a. Calculate the full-employment values of the real interest rate, the price level, consumption, and...
-
Does the effective interest method ensure that bond liability is carried on the balance sheet at present value throughout its life? Upon what assumption does the answer to this ques tion depend?
-
The 2007 Intel Annual Report can be found at the following Web site: www.prenhall.com/ fraser. (a) Using the Intel Annual Report, calculate key financial ratios for all years presented. (b) Using the...
-
S. Gibson is employed at an annual salary of $28,353 paid semi-monthly. The regular workweek is 38 hours. (a) What is the regular salary per pay period? (b) What is the hourly rate of pay? (c) What...
-
The Dorilane Company produces a set of wood patio furniture consisting of a table and four chairs. The company has enough customer demand to justify producing its full capacity of 2,000 sets per...
-
What is an aqueous solution? What is the difference between the solute and the solvent?
-
What is the molarity of a solution containing 55.8 g of MgCl 2 dissolved in 1.00 L of solution? a) 55.8 M b) 1.71 M c) 0.586 M d) 0.558 M
-
Why is the subordinate/equity tranche of a CDO not rated?
-
1. What was the most effective group (or team) that you have been a member of? a. What made that group so effective, what factors influenced the group's effectiveness? b. What would you change about...
-
What are some of the differences between a job that provides meaningful work, and one that provides prestige? If money is selected as the prime value a job seeker decides to pursue, what other values...
-
StarPac is a mass producer and market leader of custom plastic containers, such as paint trays and soda bottles. The company is based in Chicago and has rejuvenated itself over the past few years....
-
Describe Materials Management within a organization, a past organization, or some organization for which you are knowledgeable. Describe the functions, the flow of information, the systems used to...
-
Emma Louise Johnson lives at 85 Overton Way, Leederville, 6978, with her husband Robert David Johnson. The couple bought the house in 2020, financing it with a loan from the Commonwealth Bank, and...
-
Hemophilia is an X-linked recessive trait in humans. If a heterozygous woman has children with an unaffected man, what is the probability of the following combinations of children? A. An affected son...
-
C- Consider the following scenario:- A supermarket needs to develop the following software to encourage regular customers. For this, the customer needs to supply his/her residence address, telephone...
-
Azulene exhibits an appreciable dipole moment, and an electrostatic potential map indicates that the five-membered ring is electron rich (at the expense of the seven-membered ring). a) In Chapter 2,...
-
Compare the heat evolved at constant pressure per mole of oxygen in the combustion of sucrose (C 12 H 22 O 11 ) and palmitic acid (C 16 H 32 O 2 ) with the combustion of a typical protein, for which...
-
From the following data at 298.15 K calculate the standard enthalpy of formation of FeO(s) and of Fe 2 O 3 (s): A(kJ mol) Fe,0;(s) + 3C(graphite) 2Fe(s) + 3cO(g) FeO(s) + C(graphite) Fe(s) + CO(g)...
-
CLASS ACTIVITY: SECTION 4 Instructions: Read the question carefully. Time limit is 1 hour 2 5 minutes. Submit the scan copy of handwritten notes, word file or excel sheet on the link provided...
-
Check my Problem 17.5A Estimating inventory by the retail method. LO 17-5 The August inventory of Hawkins Company had a cost of $71,000 and a retail value of $106.000. During August, merchandise was...
-
Linda purchased a cottage in 1984 for $62,000; on February 22, 1994, it was valued at $126,000. On her 1994 tax return, Linda elected to make use of her remaining lifetime capital gains exemption in...

Study smarter with the SolutionInn App


