How many types of hybrid orbitals do we use to describe each molecule? a. NO5 b. CH5NO
Question:
How many types of hybrid orbitals do we use to describe each molecule?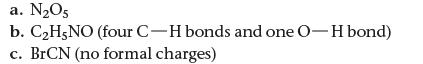
Transcribed Image Text:
a. N₂O5 b. C₂H5NO (four C-H bonds and one O-H bond) c. BrCN (no formal charges)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 85% (7 reviews)
a ...View the full answer

Answered By

Rinki Devi
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions.
Hi there! Are you looking for a committed, reliable, and enthusiastic tutor? Well, teaching and learning are more of a second nature to me, having been raised by parents who are both teachers. I have done plenty of studying and lots of learning on many exciting and challenging topics. All these experiences have influenced my decision to take on the teaching role in various capacities. As a tutor, I am looking forward to getting to understand your needs and helping you achieve your academic goals. I'm highly flexible and contactable. I am available to work on short notice since I only prefer to work with very small and select groups of students.
I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and helped them achieve great subject knowledge.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Describe the various types of hybrid orbitals and how they are used to describe the bonding in alkanes, alkenes, and alkynes. How does hybridization explain that in allene, CH 2 =C=CH 2 , the two CH...
-
An atom in a molecule has two bonds to other atoms and one lone pair. What kind of hybrid orbitals do you expect for this atom? Describe how you arrived at your answer.
-
A school computer technician who was remotely accessing the hard drives of school-issued laptops for routine maintenance found sexually explicit images of a Grade 10 student on a high school...
-
Jeremiah Wedgewood, the CFO, is adamant that the company needs to move ahead with the new division. While Josey also thinks that creating a new division with a new product line is a good idea, she is...
-
Renfree Mines, Inc., owns the mining rights to a large tract of land in a mountainous area. The tract contains a mineral deposit that the company believes might be commercially attractive to mine and...
-
In each of the following scenarios, suppose that the two nations are the only trading nations in the world. Given inflation and the change in the nominal exchange rate, which nations goods become...
-
What pattern emerges? lop4
-
Tempe Office Services and Supplies (TOSS) provide various products and services in the Tempe Research Park, home to numerous high-tech and bio-tech companies. Making color copies is one of its most...
-
4. Caron Manufacturing has the capacity to produce 20,000 units per year. Its predicted operations for the year are as follows: Sales (15,000 units @ $50 each) $750,000 Manufacturing costs: Variable...
-
George Pharmacy is a pharmaceutical salesman who has been very successful at his job in the last few years. Unfortunately, his family life has not been very happy. Three years ago, his only child,...
-
The most stable forms of the nonmetals in groups 4A, 5A, and 6A of the second period are molecules with multiple bonds. Beginning with the third period, the most stable forms of the nonmetals of...
-
The results of a molecular orbital calculation for NH 3 are shown here. Examine each of the orbitals and classify them as bonding, antibonding, or nonbonding. Assign the correct number of electrons...
-
Given that x is a hypergeometric random variable with N = 10, n = 5, and r = 7: a. Display the probability distribution for x in tabular form. b. Compute the mean and variance of x. c. Graph p(x),...
-
What is the essential objective that scientists should pursue by striving to remove personal biases, a priori commitments, and emotional involvement from their investigations about the world?...
-
c) Critically review the use of ROA (Return on Assets) as an indicator for your purposes of how a company's resources are used to generate wealth, and how different companies might measure it in...
-
Find the volume of the solid obtained by rotating the region bounded by the given curves about the specified line. Sketch the region, the solid and a typical disk or washer. -2x 3. y = ex, y = 0, x =...
-
2. Given the list of scores: Score1 = [ 10, 40, 50, 54, 55, 59, 63, 65, 70, 71, 75, 77, 79, 80, 99] The one-sample T-test is used to test whether the mean of Score1 is statistically different from...
-
Find the area of the triangle having the given measurements. Round to the nearest square unit. 13) C=100, a 3 yards, b = 8 yards Use Heron's formula to find the area of the triangle. Round to the...
-
Using Table 1.3, determine which is the more electropositive element: sodium or aluminum, carbon or nitrogen, carbon or silicon. Table 1.3 Table 1.3 Valence Electrons of the First 18 Elements Group I...
-
Use a calculator to evaluate the expression. Round your result to the nearest thousandth. V (32 + #)
-
The 2014-T6 aluminum rod has a diameter of 0.5 in. and is lightly attached to the rigid supports at A and B when T 1 = 70°F. Determine the force P that must be applied to the collar so that, when...
-
The 2014-T6 aluminum rod has a diameter of 0.5 in. and is lightly attached to the rigid supports at A and B when T 1 = 70°F. If the temperature becomes T 2 = 10°F, and an axial force of P =...
-
The force P is applied to the bar, which is made from an elastic perfectly plastic material. Construct a graph to show how the force in each section AB and BC (vertical axis) varies as P (horizontal...
-
Fredrickson Technologies last year paid a dividend of $2.4 per share. The company plans to keep these dividend payments fixed for the indefinite future. Assume that Fredrickson's equity cost of...
-
(Solving for n) How many years will it take for $520 to grow to $993.05 if it's invested at 6 percent compounded annually? Round Answer One Decimal Place
-
What would the planned shortage dollars be for the coat department if the planned net sales are $1,770,000 and the planned shortage percent is 1.9%

Study smarter with the SolutionInn App


