Identify the polyatomic ion and its charge in each compound: KNO 2 , CaSO 4 , Mg(NO
Question:
Identify the polyatomic ion and its charge in each compound: KNO2, CaSO4, Mg(NO3)2.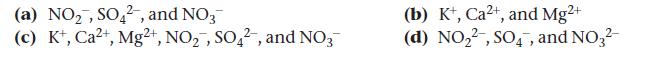
Transcribed Image Text:
(a) NO₂, SO42, and NO3 (c) K+, Ca2+, Mg2+, NO₂, SO42, and NO3 (b) K+, Ca²+, and Mg²+ (d) NO₂2, SO4, and NO3²-
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
a Only this ...View the full answer

Answered By

Kainat Shabbir
i am an experienced qualified expert with a long record of success helping clients overcome specific difficulties in information technology, business and arts greatly increasing their confidence in these topics. i am providing professional services in following concerns research papers, term papers, dissertation writing, book reports, biography writing, proofreading, editing, article critique, book review, coursework, c++, java, bootstarp, database.
5.00+
184+ Reviews
255+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
When magnesium metal is burned in air (Figure 3.6), two products are produced. One is magnesium oxide, MgO. The other is the product of the reaction of Mg with molecular nitrogen, magnesium nitride....
-
When a pure substance is placed in contact with water, there are three possible outcomes. The substance may do nothing that is, the substance does not dissolve and no visible change takes place. The...
-
Write a policy statement as the HR director stating whether or not office romantic relationships are allowed. If so, under what circumstances? What theoretical ethical perspective did you use to...
-
Melissa buys an iPod for $120 and gets consumer surplus of $80. a. What is her willingness to pay? b. If she had bought the iPod on sale for $90, what would her consumer surplus have been? c. If the...
-
What is the payback period for the following set of cash flows? Year Cash Flow 0 ....................... -$3,400 1 ....................... 1,200 2 ...................... 1,500 3...
-
A bank branch located in a commercial district of a city had the business objective of improving the process for serving customers during the noon-to-1:00 P.M. lunch period. The waiting time (defined...
-
Kayya Co. piircbases and inslalls a niaebine on .laniiary I, 2014, al a (olal cost of $ 105,000. Straight-line dcprccialion is laken each year for four years assinning a seven-year life and no...
-
An air duct heater consists of an aligned array of electrical heating elements in which the longitudinal and transverse pitches are SL = ST = 24 mm. There are 3 rows of elements in the flow direction...
-
The cash account for Norwegian Medical Coat Apr 30 indicated a balance of $14,090. The bank statement indicated a balance of 516,040 on April 30. Comparing the bank statement and the accompanying...
-
Problem 3-3B Preparing adjusting entries, adjusted trial balance, and financial statements A1 P1 P2 P3 Following is the unadjusted trial balance for Alonzo Institute as of December 31, 2015, which...
-
Name the compound Li 2 Cr 2 O 7 .
-
Explain how to write a formula for an ionic compound given the names of the metal and nonmetal (or polyatomic ion) in the compound.
-
How close should the supervisor be to the activity?
-
Richmond Clinic has obtained the following estimates for its costs of debt and equity at different capital structures: What is the firms optimal capital structure? (Hint: Calculate its corporate cost...
-
Suppose a sample yields estimates \(\widehat{\theta}_{1}=5, \widehat{\theta}_{2}=3\), se \(\left[\widehat{\theta}_{1} ight]=2\), and se \(\left[\widehat{\theta}_{2} ight]=1\) and the correlation...
-
Helium expands in a nozzle from \(0.8 \mathrm{MPa}, 500 \mathrm{~K}\), and negligible velocity to \(0.1 \mathrm{MPa}\). Calculate the throat and exit areas for a mass flow rate of \(0.34 \mathrm{~kg}...
-
The air in an automobile tire with a volume of \(0.015 \mathrm{~m}^{3}\) is at \(30^{\circ} \mathrm{C}\) and \(140 \mathrm{kPa}\) (gage). Determine the amount of air that must be added to raise the...
-
Convex Productions has just received a contract to film a commercial video that will air during a major sporting event in North America, and then be available on-demand through banner advertisements...
-
Two genes in tomatoes are 61 mu apart; normal fruit (F) is dominant to fasciated (flattened) fruit (f), and normal numbers of leaves (Lf) is dominant to leafy (lf). A true-breeding plant with normal...
-
Refer to the table to answer the following questions. Year Nominal GDP (in billions) Total Federal Spending (in billions) Real GDP (in billions) Real Federal Spending (in billions) 2000 9,817 578...
-
Predict the bond angles for all bonds in the following compounds: a) CH 3 CH 2 OH b) CH 2 O c) C 2 H 4 d) C 2 H 2 e) CH 3 OCH 3 f) CH 3 NH 2 g) C 3 H 8 h) CH 3 CN
-
Is the following statement correct? If not rewrite it so that it is correct. The superscript zero in H o f means that the reactions conditions are 298.15 K.
-
Why is it valid to add the enthalpies of any sequence of reactions to obtain the enthalpy of the reaction that is the sum of the individual reactions?
-
Prepare a 2017 T1 Return given the following information: Complete the T1 Income Tax Return and any relevant Schedules. Name: Mr. Henry Connor Age: 58 Years Old Province: Saskatchewan Gross Salary...
-
Date Aug 1 16 10 19 28 31 Activities Beginning inventory Purchase Sales Purchase Purchase Sales Units Acquired at Cost Units Sold at Retail 30 units @ $150 per unit 20 units @ $155 per unit 35 units...
-
Polaski Company manufactures and sells a single product called a Ret. Operating at capacity, the company can produce and sell 42,000 Rets per year. Costs associated with this level of production and...

Study smarter with the SolutionInn App


