Is a spontaneous redox reaction obtained by pairing any reduction half-reaction with one listed above it or
Question:
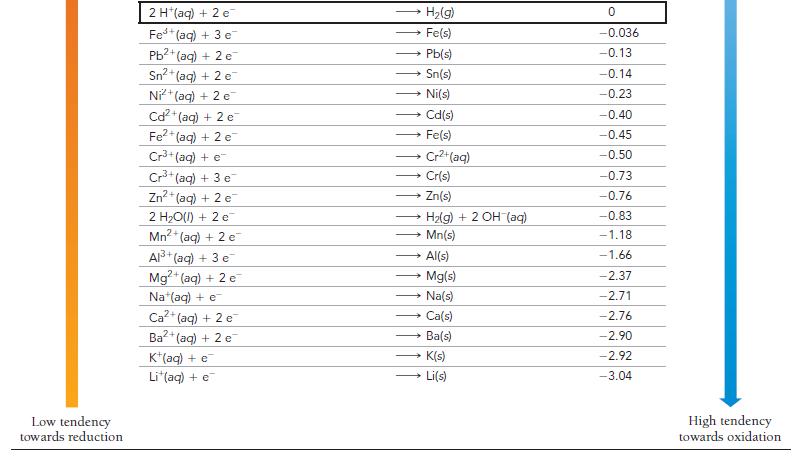
Is a spontaneous redox reaction obtained by pairing any reduction half-reaction with one listed above it or with one listed below it in Table 20.1?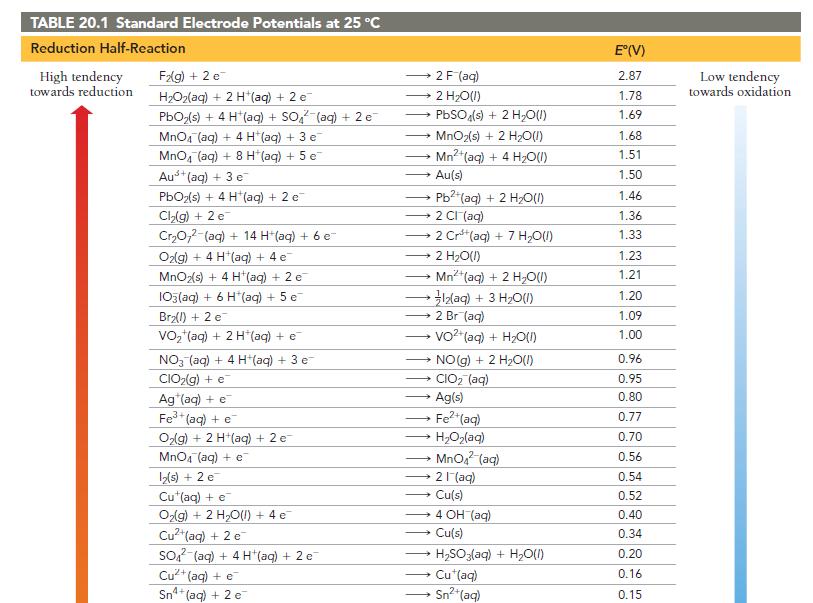
Transcribed Image Text:
TABLE 20.1 Standard Electrode Potentials at 25 °C Reduction Half-Reaction High tendency towards reduction F₂(g) + 2 e H₂O₂(aq) + 2 H+ (aq) + 2 e PbO₂(s) + 4 H (aq) + SO₂ (aq) + 2 e MnO4 (aq) + 4 H*(aq) + 3 e MnO₂ (aq) + 8 H+ (aq) + 5 e Aus+ (aq) + 3 e PbO₂(s) + 4 H*(aq) + 2 e Cl₂(g) + 2 c Cr₂O72-(aq) + 14 H+ (aq) + 6 e O2(g) + 4 H(aq) + 4 e MnO₂(s) + 4 H*(aq) + 2 e 103(aq) + 6 H*(aq) + 5 e Br₂(l) + 2 e VO₂ (aq) + 2 H+ (aq) + e NO3(aq) + 4 H+ (aq) + 3 e CIO₂(g) + e Ag (aq) + e Fe³+ (aq) + e O₂(g) + 2 H+(aq) + 2 c MnO4 (aq) + e 12(s) + 2 e Cu (aq) + e O₂(g) + 2 H₂O(l) + 4 e Cu²+ (aq) + 2 e SO² (aq) + 4 H(aq) + 2 e Cu²+ (aq) + e Sn4+ (aq) + 2 e 2 F (aq) → 2 H₂O(l) → → 2 CI (aq) PbSO4(s) + 2 H₂O(l) MnO₂(s) + 2 H₂O(1) Mn²+ (aq) + 4H₂O(1) Au(s) → 2 H₂O(l) → Pb2+ (aq) + 2 H₂O(l) — Mnt(aq) + 2 H,O(1) * 3lz(aq) + 3 H2O(I) →→→2 Br (aq) 2 Cr³+ (aq) + 7 H₂O(l) → → NO(g) + 2 H₂O(l) CIO₂ (aq) → Ag(s) VO²+ (aq) + H₂O(1) → - → HyOz(aq) Fe²+ (aq) → Cu(s) MnO₂ (aq) 21 (aq) →→→ 4 OH (aq) → Cu(s) H₂SO3(aq) + H₂O(l) Cu (aq) Sn²+ (aq) E°(V) 2.87 1.78 1.69 1.68 1.51 1.50 1.46 1.36 1.33 1.23 1.21 1.20 1.09 1.00 0.96 0.95 0.80 0.77 0.70 0.56 0.54 0.52 0.40 0.34 0.20 0.16 0.15 Low tendency towards oxidation
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
Look for pairings where the reduction potential of the halfreaction to be reduced is greater more po...View the full answer

Answered By

Lilian Nyambura
Hi, am Lilian Nyambura, With extensive experience in the writing industry, I am the best fit for your writing projects. I am currently pursuing a B.A. in Business Administration. With over 5 years of experience, I can comfortably say I am good in article writing, editing and proofreading, academic writing, resumes and cover letters. I have good command over English grammar, English Basic Skills, English Spelling, English Vocabulary, U.S. English Sentence Structure, U.K. or U.S. English Punctuation and other grammar related topics. Let me help you with all your essays, assignments, projects, dissertations, online exams and other related tasks. Quality is my goal.
4.80+
378+ Reviews
750+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-6. On December 12, Irene purchased the building where her store is located. She paid...
-
Carol Harris, Ph.D, CPA, is a single taxpayer and she lives at 674 Yankee Street, Durham, NC 27409. Her Social Security number is 793-52-4335. Carol is an Associate Professor of Accounting at a local...
-
Case Study: Quick Fix Dental Practice Technology requirements Application must be built using Visual Studio 2019 or Visual Studio 2017, professional or enterprise. The community edition is not...
-
For the car suspension shown in Figure determine the differential equation, transfer function and state space model. Plot the position of the car and the wheel after the car hits a unit bump (that...
-
Gilbert, Inc., which uses a process costing system, makes a chemical used as a food preservative. The manufacturing process involves Departments A and B. the company had the following total costs and...
-
For each of the following situations, decide what sampling method you would use. Provide an explanation of why you selected a particular method of sampling. a. A large automotive company wants to...
-
21. Repeat Problem 18 assuming that the volatility of gold is 20% and that once opened, the mine can be costlessly shut down once, and then costlessly reopened once. What is the value of the mine?...
-
You won a free ticket to see a Bruce Springsteen concert (assume the ticket has no resale value). U2 has a concert the same night, and this represents your next-best alternative activity. Tickets to...
-
Data for three business units is shown below. Determine the unknowns. (Round return on sales and investment turnover to 2 decimal places, e.g. 15.25 and all other answers to 0 decimal places, e.g....
-
Which reaction occurs at the cathode of an electrolytic cell containing a mixture of molten KCl and ZnCl 2 ? a) K(s)K+ (1) + e b) K (1) + e K(s) c) Zn+(1) + 2e Zn(s) d) 2 CI (1) Cl(g) + 2 e-
-
Describe the standard hydrogen electrode (SHE) and explain its use in determining standard electrode potentials.
-
If you created an SQL query to list the patient number and the session date, what type of join should you use? a. PLAIN JOIN b. OUTER JOIN c. INNER JOIN d. LEFT JOIN Session Num 27 28 29 30 31 32 33...
-
A release has been planned with 5 sprints. The team, for the sake of convenience, has decided to keep the sprint duration open. Depending on how much they commit and achieve, they decide to wrap up...
-
Task 3: Reach-truck management 3 Explain why battery-powered reach truck activities at PAPFS are unsatisfactory. Note: You should support your answer, where applicable, using relevant information...
-
Exercise 6: Black Pearl, Inc., sells a single product. The company's most recent income statement is given below. Sales $50,000 Less variable expenses Contribution margin Less fixed expenses Net...
-
Your maths problem x+3x-3
-
Spencer is a 10-year-old boy who has been living in a family-style therapeutic group home for one year. He was removed from his mother's care due to neglect from her drug use and the resulting legal...
-
Dorsey Co. has expanded its operations by purchasing a parcel of land with a building on it from Bibb Co. for $255.000. The appraised value of the land is $60,000, and the appraised value of the...
-
On January 1, 2018, Khalid Ltd., which follows IAS 17, entered into an eight-year lease agreement for three dryers. Annual lease payments for the equipment are $28,500 at the beginning of each lease...
-
(a) Water flows out of a shower in a typical house at a rate of 20 liters/min. If this shower is fed through a pipe that is 2.0 cm in diameter and 15 m from the high-pressure water tank, what is the...
-
Collapsing submarines. The collapse depth of a typical military submarinethat is, the depth at which the submarine would be crushed by the force due to water pressureis 700 m. If the pressure on the...
-
Measuring air speed. An airplane pilot must always know her speed relative to the surrounding air. Air speed that is too low can cause the plane to stall, whereas air speed that is too high can cause...
-
What is the Macaulay duration of a bond with a coupon of 6.6 percent, seven years to maturity, and a current price of $1,069.40? What is the modified duration? (Do not round intermediate...
-
"Tell me something you know today that you did not know yesterday" about 3D Animation Justify by citing 2 or more resources from this course.
-
Warrants exercisable at $20 each to obtain 50,000 shares of common stock were outstanding during a period when the average market price of the common stock was $25. Application of the treasury stock...

Study smarter with the SolutionInn App


