List all the possible products for each alkane substitution reaction. a. CH4 + Cl b. CH3CHBr +
Question:
List all the possible products for each alkane substitution reaction.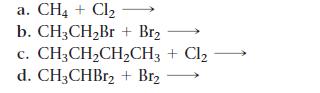
Transcribed Image Text:
a. CH4 + Cl₂ b. CH3CH₂Br + Br₂ C. CH3CH₂CH₂CH3 + Cl₂ d. CH3CHBr2 + Br₂
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (6 reviews)
a H3C H C1C1 methane dichlorine H3CC1 chloromethane HC1 ...View the full answer

Answered By

SUMAN DINDA
I LIKE TO TEACH STUDENTS. SO, I START MYSELF AS A PRIVATE TUTOR. I TEACH STUDENTS OF DIFFERENT CLASSES. I HAVE ALSO DONE BACHELOR OF EDUCATION DEGREE(B.ED). DURING THIS COURSE I HAD TO TEACH IN A SCHOOL. SO I HAVE A GOOD EXPERIENCE IN TEACHING.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
List all the possible products for each alkane substitution reaction. a. CH3CH3 + Br b. CH3CHCH3 + Cl c. CHCl + Br d. CH3-CH-CH3 + Cl 1 CH3
-
List all the possible bonds that can occur between the elements P, Cs, O, and H. Predict the type of bond (ionic, covalent, or polar covalent) one would expect to form for each bond.
-
List all the possible subshells and orbitals associated with the principal quantum number n, if n = 6.
-
In January 2021 Karl sells a one-quarter interest in a chattel for 2,500. On the date of this sale, the remaining three-quarters interest is valued at 8,500. The chattel had cost Karl 3,850 in...
-
Hutson Doubleday Company had sales of $3,948,340 and a gross margin of $1,859,260. Hutson had beginning inventory of $53,420 and ending inventory of $62,640. Required: Round answers to one decimal...
-
Some Japanese companies have a policy of rotating their managers among different managerial jobs. In contrast, North American managers are more likely to specialize in a certain area (e.g., finance...
-
What are incomparable outcomes in the context of international performance evaluations? Give a suitable example. LO.1
-
The beginning inventory and data on purchases and sales for a three-month period are shown in Problem 7-1B. Instructions 1. Record the inventory, purchases, and cost of merchandise sold data in a...
-
urgent pleasee Complete the following table. Show solutions correct to two derimsl minang
-
Name each alkene. a. CH,=CHCH,CH, CH3 CH3 T T CH3-CH-C=CH-CH3 CH,=HCCHCH,CH, CH3 b. c. CH3 CH I CH3 CH3 I d. CH3-CH-CH=C-CH3 CH - CH3
-
Complete and balance each hydrocarbon combustion reaction. a. CH,CH,CH,CH, + O, b. CH=CHCH3 + 02 c. CH=CCHCH3 + O
-
Refer to Example 7 concerning scanners. The maximum attenuation has a normal distribution with mean \(10.1 \mathrm{~dB}\) and standard deviation \(2.7 \mathrm{~dB}\). (a) What proportion of the...
-
Evaluation a. Evaluate the effectiveness of social media marketing campaign for instagram, facebook and pintrest ?based on your KPIs for example account reached, content reached, likes, shares,...
-
A study was performed at a university to analyze whether the preference for hamburgers or fried chicken is related to the gender of the student. This table lists the results of the study. At a =...
-
A 20-lb homogeneous box has tipped and is resting against a 40-lb homogeneous box as shown in figure attached. The coefficient of friction between box A and the floor is 0.7, and between box B and...
-
The Taylor series for natural logarithm (with base e) In(1+r) is In(1+2) -(-1)+1 for <1. (a) Write a user-defined function using loop that determines In(1+x) using the above Taylor series. Your...
-
Question 1: [up to 4 pts] Suppose that a = 1, a2 = 2, a3 = = 3, and an = an-3 for all n 4. If an integral with respect to y is used to find the area of R, what should the upper limit of integration...
-
Using the information provided in Problem F-3, illustrate Sue's indifference curve, with water on the horizontal axis and soft drinks on the vertical axis.
-
Define deferred revenue. Why is it a liability?
-
Your university radio station has a 5.0-kW radio transmitter that broadcasts uniformly in all directions; listeners within 15 km have reliable reception. You want to increase the power to double that...
-
A parallel-plate capacitor has circular plates with radius 50.0 cm and spacing 1.0mm. A uniform electric field between the plates is changing at the rate of 1.0 MV/ms. Find the magnetic field between...
-
Youre engineering a new cell phone, and youd like to incorporate the antenna entirely within the phone, which is 9 cm long when closed. The antenna is to be a quarter-wavelength long a common design...
-
Suppose I have computed the cost of carbon per mile for my car at 0 . 0 1 2 per mile. Assume that the interest rate is 4 % and that I drive the car 2 8 , 0 0 0 miles per year. What is the present...
-
Imagine that in stable growth period, the firm earns ROIC of 10% and has after tax EBIT of 200 and reinvestment $ of 40. What is the steady state growth rate? 20% O 10% 2%
-
Tanner-UNF Corporation acquired as a long-term investment $160 million of 5.0% bonds, dated July 1, on July 1, 2021. Company management has the positive intent and ability to hold the bonds until...

Study smarter with the SolutionInn App


